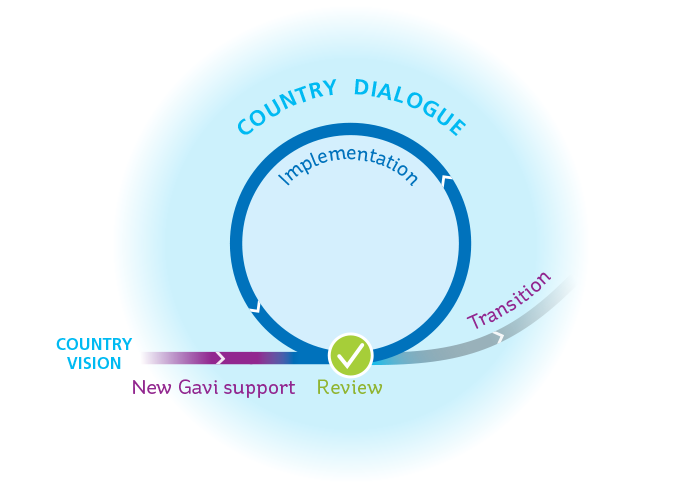
Overview of Gavi’s grant cycle
Eligible countries can implement a portfolio of Gavi support, consisting of;
- health system strengthening (HSS) support
- vaccine support
- cold chain equipment optimisation platform (CCEOP) support
- targeted country assistance (TCA)
Our support aims to assist countries in advancing their national immunisation plans and improving immunisation coverage and equity in a sustainable way.
Elearnings
Accessible to everyone.
NEW GAVI SUPPORT
Over time, countries may request new Gavi support to be added to their existing portfolio. We strongly encourage them to base such requests on their national vision, and to align Gavi support with their own planning and financial cycles.
Requests for new support are reviewed by the Independent Review Committee (IRC). Once a request is approved, the funds or vaccines are sent to the country, marking the beginning of the implementation phase.
IMPLEMENTATION
To renew their portfolio of Gavi support on a yearly basis, countries must regularly submit monitoring and reporting data. This includes an annual joint appraisal review of how Gavi-supported programmes are progressing, as well as regular reporting against set indicators, details of which can be found in Gavi’s support guidelines. Regular reporting is key to monitoring the performance of Gavi support, and informs Gavi’s decisions on disbursements and continued support.
Applying or renewing support?
Transition
Gavi works closely with countries to ensure that investments support the long-term programmatic and financial sustainability of their immunisation programme. The ultimate goal is for countries to transition out of our support once they have robust systems and decision-making processes in place.
Gavi works closely with countries to ensure that investments support the long-term programmatic and financial sustainability of their immunisation programme. The ultimate goal is for countries to transition out of our support once they have robust systems and decision-making processes in place.
Deadlines
Countries should aim to meet a deadline that fits with their own timeframes for implementation. If a country misses a submission deadline, the request for new support or renewal will be reviewed in the following round.
All requests for renewal of vaccine support must be submitted by 15 May each year. In addition, countries should submit a joint appraisal report each year.
Countries should aim to meet a deadline that fits with their own timeframes for implementation. If a country misses a submission deadline, the request for new support or renewal will be reviewed in the following round.
All requests for renewal of vaccine support must be submitted by 15 May each year. In addition, countries should submit a joint appraisal report each year.
Deadlines to submit requests for new support |
|---|
25 January 2024 |
18 April 2024 |
23 September 2024 |
22 January 2025 |
1 May 2025 |
16 September 2025 |
Welcome to Need to Know, a digital newsletter for countries and partners delivering the latest on Gavi policies, guidance, and other programmatic updates.
Access the previous editions using the links below.
Need to Know – 2025
Topics include:
- Summary outcome from the Board meeting
- New product category ‘TPVS’ added to the Gavi CCEOP to help reach last mile populations
- Request for Feedback: Gavi Funding Policy Review (FPR) Consultation
- Insights from meeting of the Independent Review Committee (IRC)
- Gavi Zero-Dose Learning Week: Advancing Evidence-Based Programming
Need to Know – 2024
Topics include:
- 2024 Gavi Funding & Process Briefing Series
- Insights from meeting of the Independent Review Committee (IRC)
- Gavi’s 5.0 learning priorities
- Zero-Dose Learning Hub semi-annual update
Topics include:
- Gavi Vaccine Funding Guidelines updated
- Last chance for requesting Innovation Top-up support is approaching!
- Gavi Board approves next five-year strategy from 2026–2030
- Insights from meeting of the Independent Review Committee (IRC)
Topics include:
- Closing of submission window for 2024 June IRC (Independent Review Committee)
- Opening of 2025 COVID-19 Programme Dose Application Window
- Insights from meeting of the Independent Review Committee (IRC)
- Key take-aways from meeting of the Alliance Partnership and Performance Team (APPT)
- Zero-Dose Learning Hub webinar series
Topics include:
- COVID-19 vaccination: seizing the opportunity for a life-course immunisation approach
- 2023 End-of-year Stock Reporting
- Closing of submission window for 2024 March IRC (Independent Review Committee), and opening of submission window for applications for 2024 June IRC
- Zero-Dose Learning Hub webinar series
Need to Know – 2023
Topics include:
- Opening of Round 1 and 2024 IRC dates
- Big Catch Up
- Monitoring and Learning (M&L)
- Celebrating 2023
- New Zero-Dose case studies series offers insights to programmatic optimisation
Topics include:
- New vaccine support available: DTP-containing boosters, hexavalent, and integration of COVID-19 vaccine (routine)
- Application deadline is coming soon!
- Equity Accelerator Funding extended to 2027
- Opening of Wave 2 of the COVID-19 Programme 2024
- Updated CCEOP requirements
- Evidence-based ZD programming: a pro-equity evidence map
- Gavi’s 2022 Annual Progress Report (APR)
Topics include:
- Opening of Round 4 for applications
- Measles/Measles Rubella
- COVID-19 Delivery Support (CDS) Reporting
- Evaluation of the Gavi Independent Review Committee
- Evidence-based ZD programming: a pro-equity evidence map
- Zero-Dose experience-sharing session (ZDLH-X2)
Topics include:
- 2023 Gavi Funding & Process Briefing Series
- Opening of Round 3 for applications
- Digital Health Information Survey
- PHEIC Status Lifting
Topics include:
- Updated: Gavi’s Co-financing, eligibility &transition thresholds
- Gavi’s HPV Vaccine Revitalisation Effort
- Updated Vaccine Funding Guidelines available
- Updated CCEOP budget templates available
- COVAX Transition and C19 Programme 2024-25
- Updated WHO SAGE Guidance
Topics include:
- 2024 Vaccine Investment Strategy (VIS) – survey to country partners
- Opening of Round 2
- 2022 End-of-year Stock Reporting
Need to Know – 2022
Topics include:
- Outcomes from the Gavi Board meeting
- Changes to Gavi Funding Policies approved by Gavi Board
- Digital Health Information strategy
- Comprehensive Vaccine Management (CVM) Approach
Topics include:
- Updated Application Templates & Guidelines available!
- Updated Programme Funding Guidelines
- Opening of Round 1 and 2023 IRC dates
- Innovation Top-Up funding
- GenderPro – Immunisation Sector Track
- Gender and Immunisation Demand
- Healthworker capacity building tools available
Topics include:
- Launch of Gavi’s first malaria vaccine programme
- Launch of Preventive Cholera Vaccination within Gavi’s Portfolio
- Gavi Vaccine Funding Guidelines updated
- New Window of COVID-19 Delivery Support Funding
- 2023 Yellow Fever Diagnostic Support Renewals
- 5.0 Joint Appraisal
- Gavi's new Fragility, Emergencies and Displaced Populations Policy
- Review outcomes of Targeted Country Assistance (TCA)
Topics include:
- Guidelines related to renewal and multi-year approval of vaccine support
- Guidelines related to Targeted Country Assistance (TCA) 2022-2025 multi-year planning
- IPV catch-up support window closes in 2022
Topics include:
- Funding Guidelines available to countries applying for Gavi support
- Updated CCEOP materials
CDS Needs Based Window – update, latest guidelines, rolling submissions
- Gavi’s Fragile, Emergencies and Refugees Policy is going through a review: have your say!
- Insights from meeting of the Independent Review Committee (IRC)
- Why gender matters
Topics include:
- Upcoming Deadlines for Stock Reporting & Vaccine Renewal Requests
Need to Know – 2021
Topics include:
- Summary outcome from the Board meeting
- Harnessing the full potential of civil society expertise – new Board-approved requirement
- Malaria Vaccine Programme Approved by the Gavi Alliance Board
- Opening of Round 1 and 2022 IRC dates
Topics include:
- HPV Vaccines: New HPV Vaccine receives WHO Prequalification
- New National Immunisation Strategy (NIS) Guidance Available
- Needs-Based Funding Window of COVID-19 Vaccine Delivery Support (CDS)
- Urban Immunisation
- The French translation of Gavi’s 2020 Annual Progress Report (APR) is now live!
Topics include:
- Launch of Community of Practice for reaching zero-dose children
- New Partnerships to identify and reach zero-dose children with immunisation services in 12 countries across the Horn of Africa and the Sahel
- Summary outcome from the Board meeting
- Needs-Based Funding Window of COVID-19Vaccine Delivery Support (CDS)
- Insights from the latest IRC meeting
- Gavi’s 2020 Annual Progress Report (APR) is now live!
Topics include:
- Equity Accelerator Funding (EAF) Available for Countries to Accelerate Efforts to Reach Zero-dose Children and Missed Communities
- EAF Application Pre-Requisites
- Accessing Equity Accelerator Funding
Topics include:
- Round 3 Opened for New Applications – Due Date 15 September
- Key take-aways from the Vaccine High Level Review Panel (HLRP): 14-15 July 2021
- Insights from meeting of the Independent Review Committee (IRC)
Topics include:
- Gavi 5.0 Application Materials now available
- Updated Vaccine Funding Guidelines released
- Opening of Supplemental Application Round with submission deadline of 1 July 2021
- New National Immunization Strategy (NIS) Guidelines to be available by end July 2021
Topics include:
- Application for Inactivated Polio Virus Second Dose Due on 4 May 2021
- Accessing the country portal for renewals
- Upcoming changes related to Gavi 5.0
- Malaria Vaccine Implementation Programme (MVIP) – Update on 2nd anniversary
- Key lessons learned from the first four years of implementation of the Cold Chain Equipment Optimization Platform (CCEOP)
Topics include:
- Upcoming Reporting Deadlines: Stock Reporting & GPF Reporting
- Round 2 Opened for New Applications - Due Date 4 May
- Requirement Waived for Countries with Expiring Comprehensive Multi-Year Plans
- Immunisation Agenda 2030 Update
- Need to Know Archive
Topics include:
- Board Approval of Additional Financing to Accelerate Progress towards Equity in Immunisation
- Gavi 5.0 Measurement Framework
- Middle Income Country Approach Approved by the Board
- Gavi Guidance to Address Gender-Related Barriers to Maintain, Restore, and Strengthen Immunisation in the Context of COVID-19
- REMINDER: OneTA Plans for 2021 TCA Support to be Submitted by EPI Managers to Gavi Secretariat by 28 February 2021
- TCV Introduction in Liberia and Zimbabwe
Need to Know – 2020
Topics include:
- rotavirus vaccine profiles supported by Gavi
- 2021 TCA guidance and support materials
- resource kit for girl-focused communications for HPV campaigns
- cyber-security risks
- insights from the latest IRC and HLRP meetings
Topics include:
- Update on Vaccine Request Form
- Country Briefings on the COVAX Indemnification Agreement and COVAX Cost-Sharing
- Communication of Cold Chain Equipment Support Ceilings.
25 NOVEMBER – (COVAX NTK)
Topics include:
- COVAX Application Materials for AMC Eligible Economies Available in French, Spanish, and Russian
- Follow-up to AMC Engagement Group Meeting - 19 November 2020
13 NOVEMBER – (COVAX NTK)
Topics include:
- COVAX Application Materials for AMC Eligible Economies Now Available
- AMC Engagement Group
Topics include:
- Round Opened for New Applications - Due Date 19 January
- Expanding partnerships to reach missed children in fragile and conflict settings
- Recalibration of strategic priorities for Gavi 5.0 in light of Covid-19 and the successful replenishment
- Using Geospatial Technologies to improve immunisation coverage and equity
Topics include:
- COVAX: Gavi Board approved funding to jumpstart support
- Guidance for countries – maintaining, restoring and strengthening
- Gavi's first ever innovation catalogue is available
- Case Study: Understanding urban challenges in Uganda
- Gavi's 2019 Annual Progress Report is out
Topics include:
- Gavi’s support in the face of COVID-19: updated programming guidance coming soon
- Learn and adapt to increase access to immunisation
- Cambodia: performance of an immunisation system under COVID-19
- Key take-aways from the Vaccine High Level Review Panel (HLRP)
- Coming soon: Gavi's 2019 Annual Progress Report
- July IRC report released
- Multi-stakeholder dialogue planning
Topics include:
- Support approved for former-Gavi countries
- PEF Targeted Country Assistance (TCA) June 2020 Reporting: COVID-19 impacts achievement rate
- Gavi Independent Review Committee Insights
- What We Learned: Supportive supervision of frontline workers case study
- COVID-19 Vaccines Global Access (COVAX) Facility
- Need to Know Archive
Topics include:
- New COVID-19 Vaccines Global Access (COVAX)
- COVID-19 & Immunisation multi-stakeholder dialogue
- 2020 Applications – Round three due 8 September
- Gavi’s new Gender Policy
- How Health Systems Immunisation Strengthening (HSIS) is changing
- Programme readiness filter removed.
Topics include:
- Exceptional measures to support countries mitigate the fiscal impact of the COVID-19 pandemic on immunisation
- Requirement Waived for Countries with Expiring Comprehensive Multi-Year Plans
- Targeted Country Assistance Flexibility
- PEF Partners Progress Reporting on Strategic Focus Areas (SFA), Targeted Country Assistance (TCA), and Post Transition Engagement in Partner Portal
- Inactivated Poliovirus Vaccine Second Dose Requests Due 15 July
- 2021 Yellow Fever Diagnostic Support Renewals.
Last updated: 13 May 2025