Gavi’s impact
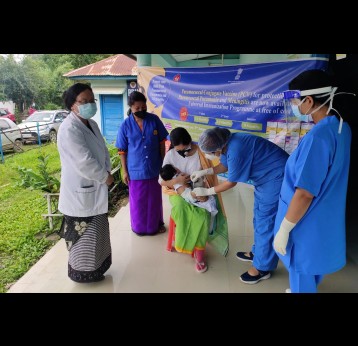
The vast majority of Gavi-supported countries have introduced pneumococcal vaccines, reaching more than 215 million children by the end of 2019 – and growing pneumococcal vaccine coverage in Gavi-supported countries above the worldwide average.
MORE THAN 215 MILLION CHILDREN REACHED
By the end of 2019, Gavi support had helped countries immunise more than 215 million children across 60 lower-income countries against pneumococcal disease.
In 2019, for the first time, pneumococcal vaccine coverage in Gavi-supported countries was higher than the worldwide average: 49% in 2019, up from 37% in 2015 and above the global average of 48%. This reflects a decade of progress and hard work by countries and Vaccine Alliance partners to support the introduction of pneumococcal vaccine into routine immunisation programmes and to scale up coverage.
Gavi contributed to the development of UNICEF and WHO’s integrated Global Action Plan for Pneumonia and Diarrhoea (GAPPD), which in 2013 set a target to reduce mortality from pneumonia in children aged under five to fewer than 3 per 1,000 live births.
PNEUMOCOCCAL ADVANCE MARKET COMMITMENT (AMC)
Pneumococcal conjugate vaccines (PCV), which protect against the main cause of pneumonia, are complex to develop and produce. In the past, they may have taken up to 15 years to reach lower-income countries. Thanks to the Vaccine Alliance, Gavi countries can access the newest vaccines at the same time as high-income countries.
In its eleventh year of implementation, the Advance Market Commitment (AMC) for pneumococcal vaccines has facilitated the procurement of a total of 161 million doses of PCV for lower-income countries, an 8% increase from 2018. The introduction of these vaccines is largely thanks to the generosity of Italy, the United Kingdom, Canada, the Russian Federation, Norway and the Bill & Melinda Gates Foundation. They have together contributed more than US$ 1.3 billioni to the Pneumococcal AMC.
ROLL-OUT ACROSS THREE CONTINENTS
Gavi aimed to support pneumococcal vaccine introductions in 45 countries by 2015. We reached this target already in November 2014, 13 months ahead of schedule. By the end of 2019, 60 Gavi-supported countries – more than 80% of those eligible to do so – had introduced pneumococcal conjugate vaccine (PCV) into their routine immunisation programmes.
Mongolia, which transitioned out of Gavi support in 2015, rolled out the vaccine in June 2016, becoming the first transitioned country to fully self-fund its pneumococcal vaccine programme; it was followed by Bhutan in January 2019. In 2020, three countries that have transitioned out of Gavi support – Indonesia, Timor-Leste and Ukraine – requested and received access to the AMC PCV price and are expected to introduce PCV into their routine immunisation programmes in the next two years.
The issue
Leading cause of pneumonia
Pneumococcal disease is caused by the Streptococcus pneumoniae bacterium. It is the leading cause of pneumonia, which kills more children each year than any other disease.
Streptococcus pneumoniae can also cause meningitis, which often leaves survivors with permanent disabilities. Another pneumococcal infection is sepsis, which can lead to amputation or death. Pneumococcal otitis media, a middle ear infection, can result in permanent deafness.
Pneumonia is the single largest infectious cause of death in children worldwide, killing more than 800,000 children aged under five in 2017 alone. Pneumonia is most prevalent in South Asia and sub-Saharan Africa.
Pneumococcal vaccines
Safe and affordable vaccines are the most effective way to prevent pneumococcal infection.
The World Health Organization (WHO) recommends that all countries introduce pneumococcal vaccines into their routine immunisation programmes, and that all children receive three doses of pneumococcal vaccine. This is particularly important in countries with high levels of pneumonia and high child mortality rates.
Gavi’s response
Pneumococcal Advance Market Commitment (AMC)
Gavi, the World Bank and donors launched the Pneumococcal Advance Market Commitment (AMC) in 2009 to stimulate the development and manufacture of new vaccines for lower-income countries. The AMC has helped countries access more affordable pneumococcal conjugate vaccines (PCV) adapted to their epidemiology. The AMC mechanism reached its sunset at the end of December 2020. The contracts in place with manufacturers will continue through 2029.
- The World Bank provided fiduciary support.
- Italy, the United Kingdom, Canada, Russia, Norway and the Bill & Melinda Gates Foundation committed US$ 1.5 billion to launch the AMC.
- Gavi contributed US$ 3.3 billion to support the cost of pneumococcal vaccines between 2009 and 2020. We also provide programmatic and administrative support.
- WHO established specific criteria for the vaccines and provided technical assistance.
- UNICEF handles vaccine procurement and distribution to countries.
The AMC gave manufacturers an incentive to invest in:
- finalising the development of pneumococcal conjugate vaccines that protect against more serotypes. In lower-income countries, these serotypes often cause disease and death.
- developing a multi-dose-vial presentation more suitable for the limited cold-chain capacity of lower-income countries.
- increasing their capacity to produce these vaccines in enough quantities to meet demand.
At the same time, the Pneumococcal AMC gave lower-income countries the assurance that enough vaccines will be available at a price they can afford over the long term. As a result, countries are better able to plan and budget for their immunisation programmes.
Vaccines supported through the Pneumococcal AMC must meet specific criteria developed by WHO. They must also receive approval by Gavi’s Independent Assessment Committee (IAC).
Three manufacturers committed to supply more than 1.65 billion doses through 2029. Through the Pneumococcal AMC, pneumococcal vaccines are available to Gavi countries at no more than US$ 3.50 per dose – less than 5% of the public price in the United States of America. By early 2019, Gavi had secured a lowest price offer from one of its pneumococcal vaccine suppliers of US$ 2.90 per dose. This price drop, the third in three years, represented a US$ 0.40 drop since January 2017.
In June 2020, a third manufacturer joined the Pneumococcal AMC, bringing more dynamism to the PCV market. The first developing country vaccine manufacturer to access the global PCV market will provide 10 million doses each year to Gavi countries at US$ 2.00 per dose. Depending on how many countries switch to the lower-cost product and the speed of country introductions, this is estimated to generate additional savings of up to US$ 50 million.
We are working with partners to ensure that supply of the vaccine remains stable. We also aim to make sure that countries that have yet to introduce PCV into their routine immunisation programmes get the support they need to do so. Another important goal is that existing programmes are sustainable over the long term.
Routine immunisation support
Gavi provides support for routine introduction and catch-up at introduction by funding the pneumococcal conjugate vaccine and injection supplies. Further, countries receive a one-time vaccine introduction grant.
As part of its commitment to the WHO/UNICEF Integrated Global Action Plan for the Prevention and Control of Pneumonia and Diarrhoea (GAPPD), Gavi requests that the use of pneumococcal vaccines be part of a comprehensive and integrated strategy alongside other related interventions, such as oral rehydration therapy; exclusive breastfeeding; zinc treatment; improvements in water, sanitation and hygiene; as well as proper nutrition.
From other sites