Two reviews, supported by the GAVI Alliance, will help key decision-makers and implementers in GAVI Alliance-supported countries to optimise the introduction of childhood vaccines against pneumococcal disease and rotavirus, the two leading causes of childhood death, by severe pneumonia and diarrhoea respectively.
The first, a review of pneumococcal conjugate vaccine (PCV) dosing schedules, demonstrated that PCV is effective across three different vaccination schedules currently in use, while the second, which looked into the rotavirus disease burden in Africa, concluded that rotavirus surveillance will be vital to successful and sustained introduction of the vaccine.
PCV Dosing Landscape Study
The “PCV Dosing Landscape Study” – led by the US Centers for Disease Control and Prevention, Johns Hopkins’ International Vaccine Access Center and the University College London’s Institute for Child Health – represents the most comprehensive review of dosing schedules to date.
Study researchers, who reviewed nearly 13,000 articles and 14 databases covering studies from 1994-2010, evaluated the relationship of a variety of outcomes to three PCV schedules: two doses in early infancy with a booster later in the first year (2+1), three doses in early infancy with no booster (3+0), and three doses in early infancy plus a later booster (3+1).
Subtle differences
Results showed that all schedules were effective in preventing disease and could help achieve major reductions in pneumococcal disease. However, the study revealed that subtle differences exist between schedules and that careful balancing of programmatic and epidemiologic contexts could yield the greatest possible benefit from PCV introduction and widespread use.
Investigators concluded that the most critical goal should be to achieve high levels of vaccine coverage as quickly as possible, regardless of the schedule used.
Outcomes from this analysis were shared with the World Health Organization’s (WHO) Strategic Advisory Group of Experts (SAGE) and helped to inform WHO’s 2012 position paper on PCV use, which now offers the 2+1 schedule as an alternative to the previously recommended schedule of 3+0 doses.
Vast potential
According to WHO estimates, the pneumococcal bacterium claimed the lives of more than half a million young children in 2008, illustrating the vast potential impact of PCV vaccines, which can be maximised with the aid of this review.
The review has been published as a supplement in the Pediatric Infectious Disease Journal (PIDJ), and can be openly accessed here.
Rotavirus Disease Burden in Africa Supplement
The Rotavirus Disease Burden in Africa supplement contains a series of articles that describe the critical role of surveillance for monitoring burden of disease, and provide a baseline against which rotavirus vaccine safety and impact can be measured following its introduction.
Heavy burden
The studies, which analysed data from more than 42,000 children under five across 20 countries, hospitalised for acute diarrhoea between 2006 and 2012, identified the heavy burden of rotavirus as a cause of diarrhoeal disease across Africa. In total, approximately 41% of these children tested positive for rotavirus, with individual rotavirus burden analyses for ten countries ranging considerably from 20% in rural western Kenya to 56% in Nigeria.
Hospital-based gastroenteritis cases positive for rotavirus
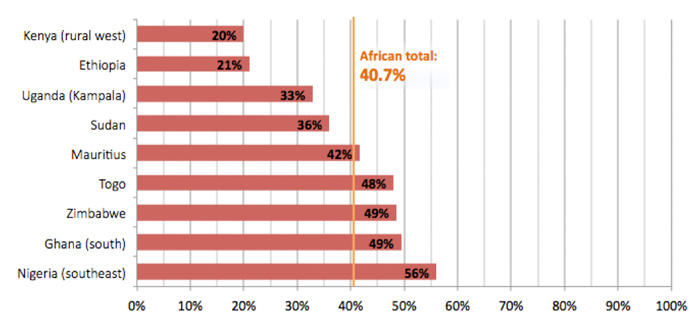
Such findings indicate that surveillance will provide useful benchmarks for countries such as the 15 that have already introduced the vaccine (11 with GAVI Alliance support), to help them monitor progress against rotavirus.
Building networks
This supplement adds to wider efforts to establish and maintain rotavirus surveillance networks in Africa and in other regions of the world. The GAVI Alliance has provided financial support to WHO to help countries build capacity for these networks, such as the Global Rotavirus Surveillance Network, which will provide valuable data for rotavirus vaccine implementation, monitoring, and evaluation efforts as additional African countries introduce rotavirus vaccines.
The full set of articles in the Pediatric Infectious Disease Journal (PIDJ) supplement can be read here.
