A smart label on vaccine vials will be vital for safely rolling out future COVID-19 vaccines
Interim results suggesting that Pfizer/BioNTech’s COVID-19 vaccine provides more than a 90% efficacy, offers hope that immunisation can be effective against COVID-19. But the need to store this vaccine at “ultra cold” temperatures could pose challenges for transportation and storage. A new smart label will help health workers tell if vaccines have been stored correctly and are safe to use.
- 12 November 2020
- 3 min read
- by Gavi Staff
The race to develop COVID-19 vaccines could soon see highly innovative new vaccines being rolled out. But with some of these novel vaccine technologies come new transportation and storage challenges. In order to remain safe and effective some, like the one developed by Pfizer/BioNTech, will need to be stored at temperatures below -70 degrees Celsius, well below the range at which most refrigerators and freezers operate. Also, these vaccines have relatively short storage times, compared to standard vaccines.
Specialised refrigeration equipment does now exist to ensure that such vaccines remain at optimised temperatures throughout the cold chain – the long and sometimes complex journey from vaccine manufacturer to clinic. However, there is always a risk that even brief exposure to higher temperatures along this journey, or at the clinic, could damage the vaccines. So, it is vital that health workers know that the vaccines are effective before they use them.
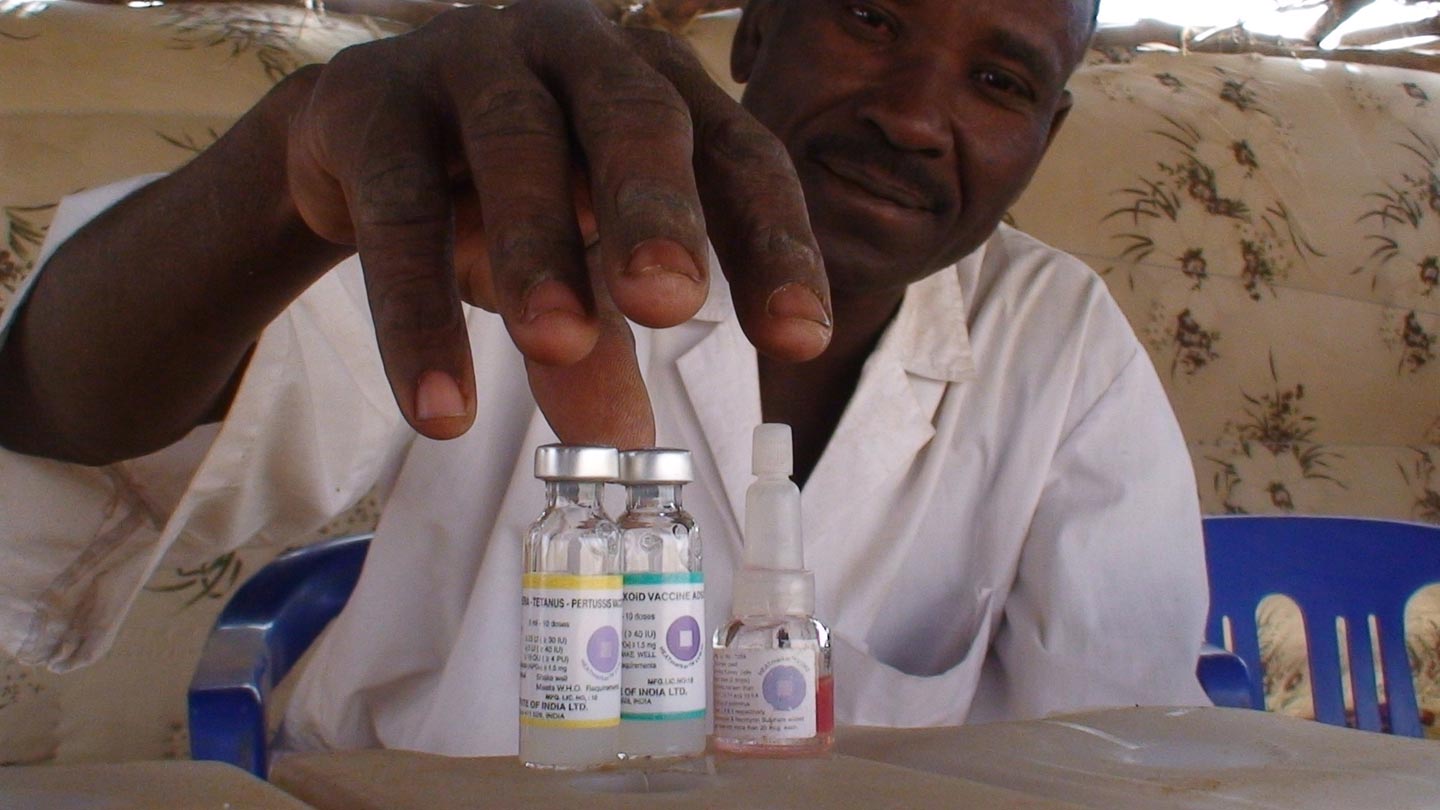
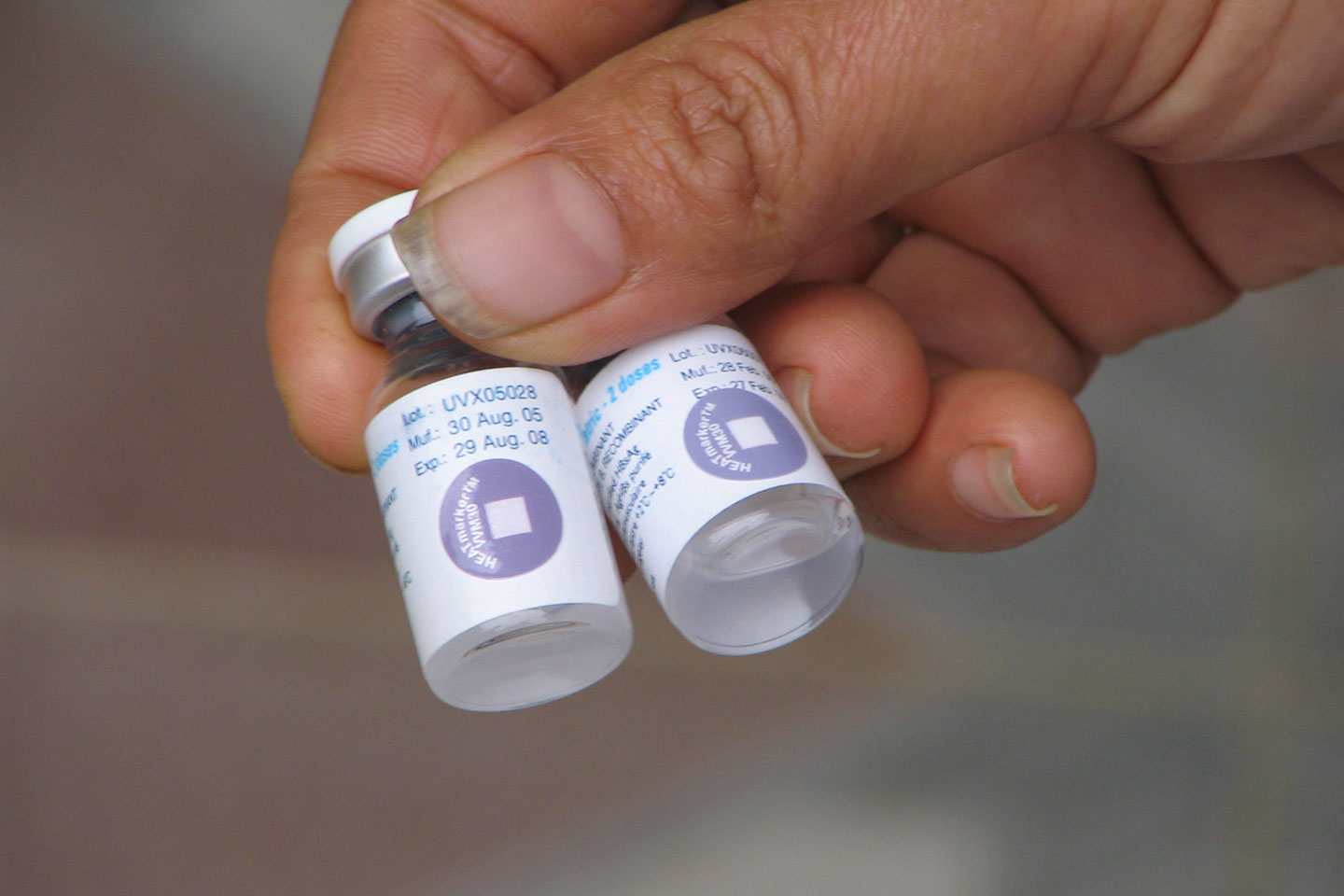
To help with this, most vaccine vials now come with a vaccine vial monitor (VVM) attached to them. These smart labels are time-temperature indicators that contain a colourless chemical which irreversibly reacts in proportion to heat and time, becoming darker to give a visual indication of cumulative heat exposure. Their design is such that once a vaccine vial has been exposed to temperatures for a period beyond their optimal storage range, the label changes in appearance to indicate the vaccine has spoiled. With one look, health workers and vaccinators can tell if a vial has been left out too long or has spoiled during the journey and discard it.
These smart labels are time-temperature indicators that contain a colourless chemical which irreversibly reacts in proportion to heat and time.
Currently, 230 of the 248 vaccines that have been pre-qualified by the World Health Organization are now required to carry a VVM. Different vaccines have different optimal storage requirements and different tolerances to high temperatures, with some, for example, able to remain stable and safe to use for several weeks after leaving the cold chain, even in relatively high ambient temperatures of 40 degrees Celsius.
In the case of many of the COVID-19 vaccines being developed, like Pfizer/BioNTech’s, early indications suggest much lower tolerance to heat exposure. This, and the requirement to store them at far lower than normal temperatures, has created challenges for the company that has been trying to develop several new categories of VVM for these vaccines. According to Temptime, for ultra cold chain (UCC) purposes the chemical reactivity required for VVMs to work would need to be at least an order of magnitude greater compared to VVMs that are currently available, because of the significantly shorter shelf life at all temperatures.
At its New Jersey research and development laboratory Temptime scientists developed and screened 20 different formulations for VVMs for ultra cold storage. Six of these were further assessed with one being chosen for pre-qualification by WHO. The company says similar efforts are also now underway for additional VVM types for other COVID-19 vaccines, which will go into final development once the specific needs are confirmed.
Photo gallery
More from Gavi Staff
Recommended for you