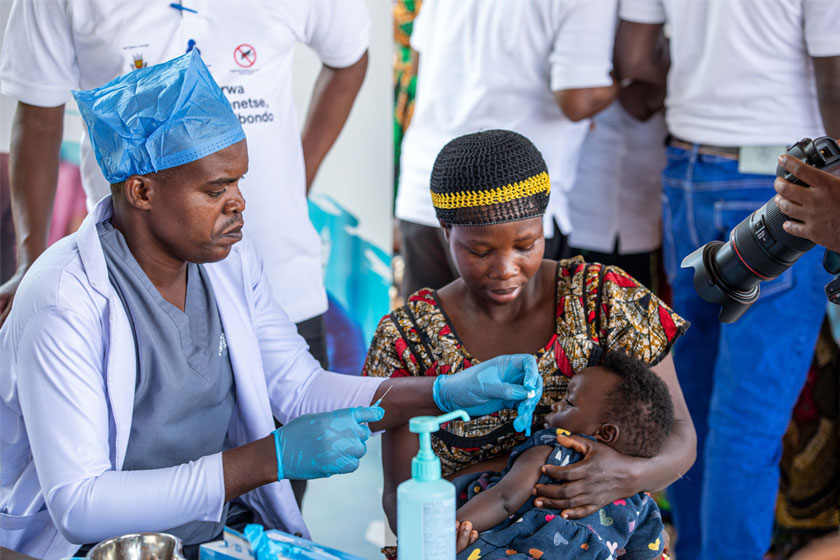
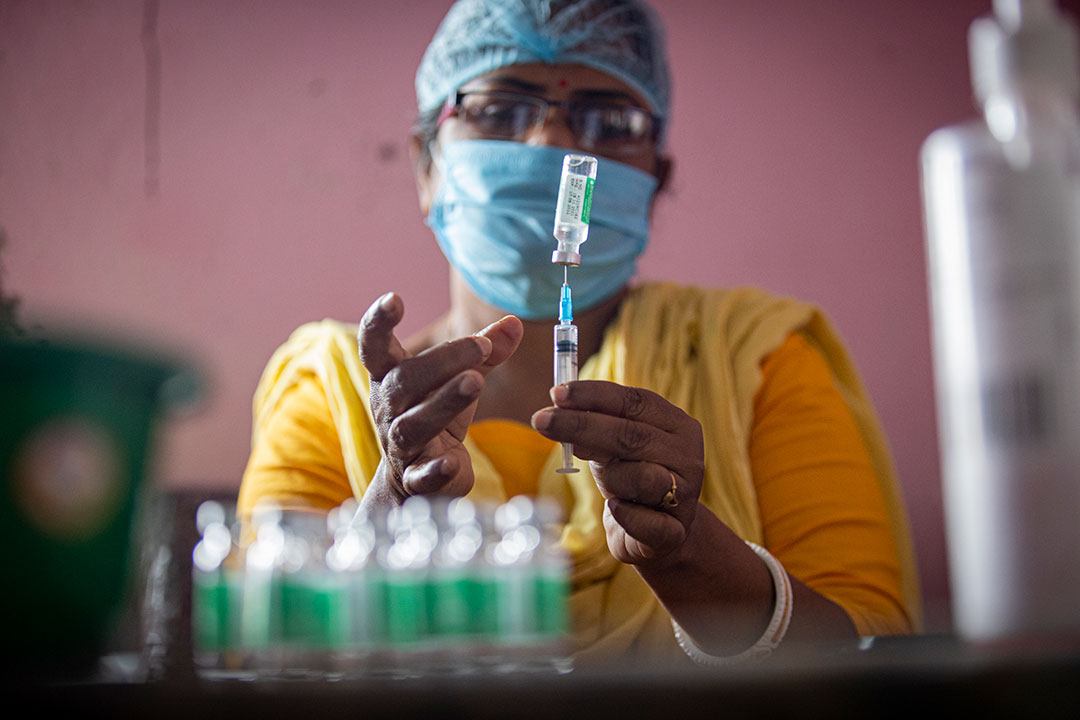
Everything you need to know about the malaria vaccine
New malaria vaccines are set to revolutionise our battle against this ancient disease. Here’s everything you need to know about these lifesavers.
- 19 January 2024
- 5 min read
- by Gavi Staff
Why do we need a malaria vaccine?
Malaria is one of the biggest global health threats, with an estimated 247 million malaria cases and 619,000 malaria deaths globally in 2021, almost all of them in Africa. On this continent, the disease is one of the biggest killers of young children, with half a million children aged under five years dying each year.
The early symptoms of malaria – headache, fever and chills – may be mild or confused with other illnesses, and so people often aren't diagnosed with malaria until it is too late. Some people recover without treatment, but the disease can be deadly – untreated, it can cause severe illness or death within 24 hours.
The road to developing a malaria vaccine has been long – about 30 years in the making – and arduous, since targeting a parasite that has a multistage life-cycle is complicated.
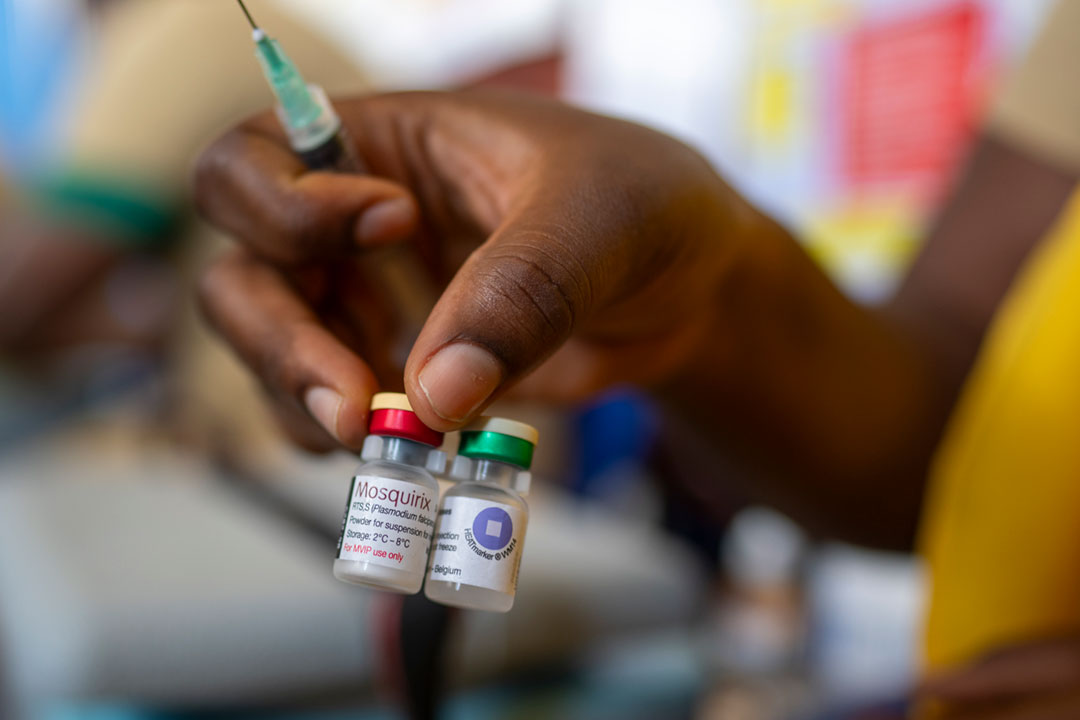
What is the RTS,S malaria vaccine?
The RTS,S malaria vaccine, also known as Mosquirix, was developed by GlaxoSmithKline (GSK) in partnership with the PATH Malaria Vaccine Initiative and is designed to target the Plasmodium falciparum parasite that causes malaria, which is spread by anopheles mosquitoes.
How does it work?
The malaria parasite has several stages in its life cycle. The vaccine targets the sporozoite, which is the infectious stage of the parasite. When a person is vaccinated, the immune system is stimulated to produce antibodies against the circumsporozoite protein, which is found on the surface of the sporozoites. If a vaccinated person is later bitten by a malaria-infected mosquito, the antibodies can neutralise the sporozoites in the bloodstream, preventing the parasite from establishing an infection.
How has the effectiveness of RTS,S vaccine been tested?
The RTS,S vaccine has been tested in rigorous clinical trials and shown to be safe and effective in children, including in those with HIV and malnutrition. Both the R21 (see final question for further detail on this vaccine) and RTS,S malaria vaccines prevent around 75% of malaria episodes when given seasonally in areas of highly seasonal transmission where malaria chemoprevention is provided.
The Malaria Vaccine Implementation Programme (MVIP), coordinated by WHO and funded by Gavi, the Global Fund to Fight AIDS, Tuberculosis and Malaria, and Unitaid, was designed to evaluate the public health use of the RTS,S vaccine in Ghana, Kenya and Malawi. Since 2019, nearly 2 million children at risk have been reached with the malaria vaccine across the three countries. The RTS,S malaria vaccine implementation has resulted in a substantial fall in severe malaria hospitalisations and a significant drop in child deaths – there was a 13% drop in all-cause mortality (i.e. not just from malaria) from use of the vaccine.
Should people continue to use bednets and indoor insecticide spraying?
Yes. As a standalone measure, the vaccine is intended as a complementary tool to other tried-and-tested effective malaria prevention and control measures. A study looking at the effect of pairing the RTS,S vaccine with bednet distribution showed that this combination was 71% effective against clinical malaria in the first 18 months and 65% effective in the second 18 months.
Who can take the vaccine?
The World Health Organization (WHO) recommends the vaccine is given to children living in malaria-endemic areas, prioritising areas of moderate and high transmission.
WHO recommends that RTS,S should be given to children from the age of five months in a schedule of four doses (vaccination programmes may choose to give the first dose at a later or slightly earlier age based on operational considerations). If children are in a high-risk area, WHO says they can be given a fifth dose a year after their fourth dose.
How is the vaccine being rolled out?
After WHO recommended the RTS,S vaccine against malaria in late 2021, Gavi's Board approved a new programme in December 2021 to support the introduction of malaria vaccines to eligible countries in sub-Saharan Africa.
In July 2023, 18 million doses of RTS,S available for 2023–2025 were allocated to 12 countries, prioritising those doses to areas of highest need, where the risk of malaria illness and death among children are highest, until vaccine supply increases to fully meet demand. As more supply becomes available, countries will expand introduction to fully meet public health needs.
In November 2023, the first shipment of 331,200 doses of RTS,S vaccine arrived in Cameroon. Demand for the malaria vaccines is significant – in 2024 alone, 20 countries in Africa plan to introduce the malaria vaccine into their childhood immunisation programmes and as part of their national malaria control strategies. Annually, at least 40–60 million doses of malaria vaccine will be needed by 2026, growing to 80–100 million doses each year by 2030.
In preparation for scaled-up vaccination, Gavi, WHO, UNICEF and partners are working with countries that have expressed interest and/or have confirmed roll-out plans on the next steps.
What is the R21 malaria vaccine?
The R21 vaccine is the second malaria vaccine recommended by WHO, which approved or 'prequalified' its use in December 2023. It works similarly to the RTS,S vaccine by targeting the sporozoite of the malaria parasite.
In areas where malaria transmission highly seasonal malaria transmission (limited to four to five months per year), the R21 vaccine reduced malaria cases by 75%. According to WHO, "This high efficacy is similar to the efficacy demonstrated when RTS,S is given seasonally."
The fact there are now two effective vaccines should ensure significant progress in working towards malaria elimination. WHO says: "There is no evidence to date showing one vaccine performs better than the other. The choice of product to be used in a country should be based on programmatic characteristics, vaccine supply and vaccine affordability."