The race to develop an Ebola vaccine
- 1 April 2018
- 11 min read

Working to prevent future devastating epidemics
The Ebola outbreak devastated countries across West Africa from 2013 to 2015. With no vaccine to curb its spread, the disease claimed more than 11,000 lives.
Scientists had researched Ebola vaccines for decades, but the lack of a commercial market removed the imperative to develop, licence and deploy such a vaccine.

Thanks to its public-private partnership model, the Alliance was able to accelerate the development of an Ebola vaccine, now available for emergency use.
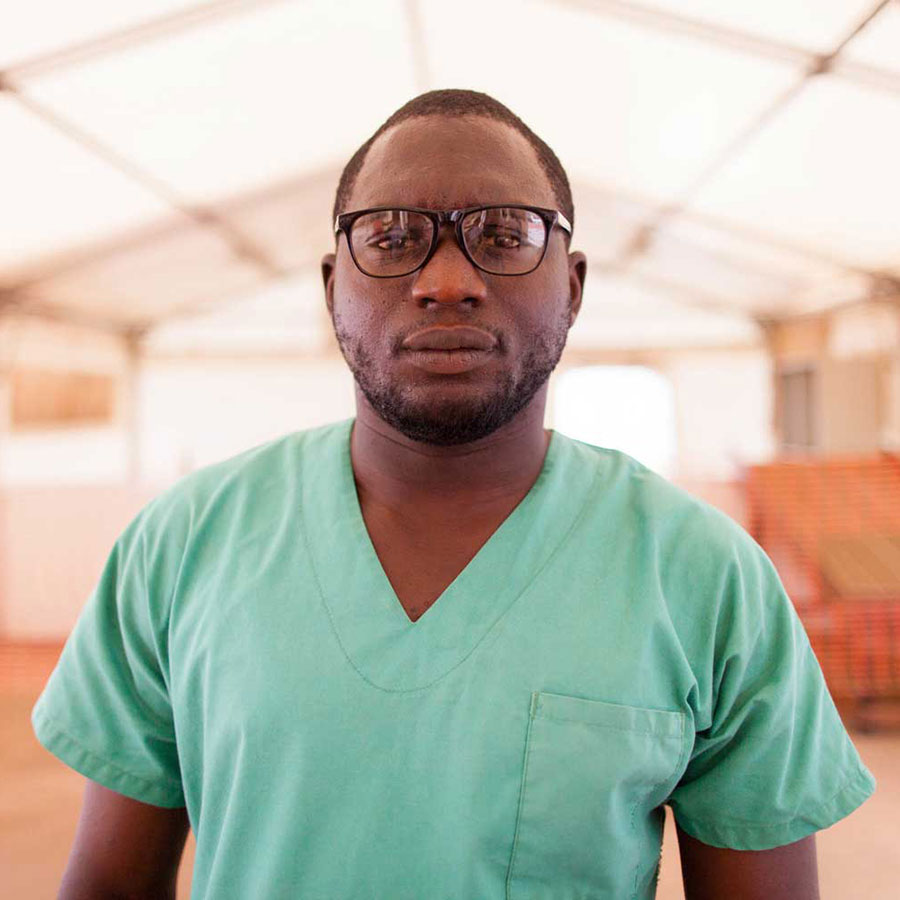
Emmanuel's story
“The families used to come and just drop them outside Redemption Hospital and then leave. Sometimes they would leave more than 15 dead bodies at once. They were dying in every home. It was quite terrible.”
In just 36 words, Emmanuel Lasanah describes the horror and devastation caused by the 2013–2015 Ebola outbreak in West Africa. Beginning in the south of Guinea, the virus rapidly spread across the country and into neighbouring Liberia and Sierra Leone. By the time the epidemic ended, it had infected almost 29,000 people, leading to more than 11,000 deaths.
Before the outbreak, Emmanuel was an outpatient supervisor at Redemption Hospital in Monrovia, Liberia. As the Ebola virus started to ravage his community, he did all he could to prevent its spread. He first became a contact tracer, leading a team of health professionals who risked their lives to trace people who had come into contact with the disease. Once an experimental vaccine against Ebola became available, expedited in a bid to curb the impact of the virus, Emmanuel was the first person in Liberia to receive it.

“The families used to come and just drop them outside Redemption Hospital and then leave. Sometimes they would leave more than 15 dead bodies at once. They were dying in every home. It was quite terrible.”
In just 36 words, Emmanuel Lasanah describes the horror and devastation caused by the 2013–2015 Ebola outbreak in West Africa. Beginning in the south of Guinea, the virus rapidly spread across the country and into neighbouring Liberia and Sierra Leone. By the time the epidemic ended, it had infected almost 29,000 people, leading to more than 11,000 deaths.
Before the outbreak, Emmanuel was an outpatient supervisor at Redemption Hospital in Monrovia, Liberia. As the Ebola virus started to ravage his community, he did all he could to prevent its spread. He first became a contact tracer, leading a team of health professionals who risked their lives to trace people who had come into contact with the disease. Once an experimental vaccine against Ebola became available, expedited in a bid to curb the impact of the virus, Emmanuel was the first person in Liberia to receive it.
Gavi model in action:
fast-tracking vaccine development
Emmanuel’s actions demonstrate how brave health professionals can help prevent the spread of infectious diseases. The experimental vaccine he received also points to another truth: that the rapid development, production and supply of new vaccines are vital to prevent such outbreaks from occurring in the first place.
If significant stocks of a viable vaccine against Ebola had been available in 2014, Emmanuel and his fellow West Africans may never have had to suffer this terrible epidemic. This is why Gavi, the Vaccine Alliance and its partners are working with both the private and public sectors to make new vaccines available before disease outbreaks can take hold. And it is the reason why the Alliance provided funding to fast-track the development of a vaccine against Ebola.
fast-tracking vaccine development
Emmanuel’s actions demonstrate how brave health professionals can help prevent the spread of infectious diseases. The experimental vaccine he received also points to another truth: that the rapid development, production and supply of new vaccines are vital to prevent such outbreaks from occurring in the first place.
If significant stocks of a viable vaccine against Ebola had been available in 2014, Emmanuel and his fellow West Africans may never have had to suffer this terrible epidemic. This is why Gavi, the Vaccine Alliance and its partners are working with both the private and public sectors to make new vaccines available before disease outbreaks can take hold. And it is the reason why the Alliance provided funding to fast-track the development of a vaccine against Ebola.
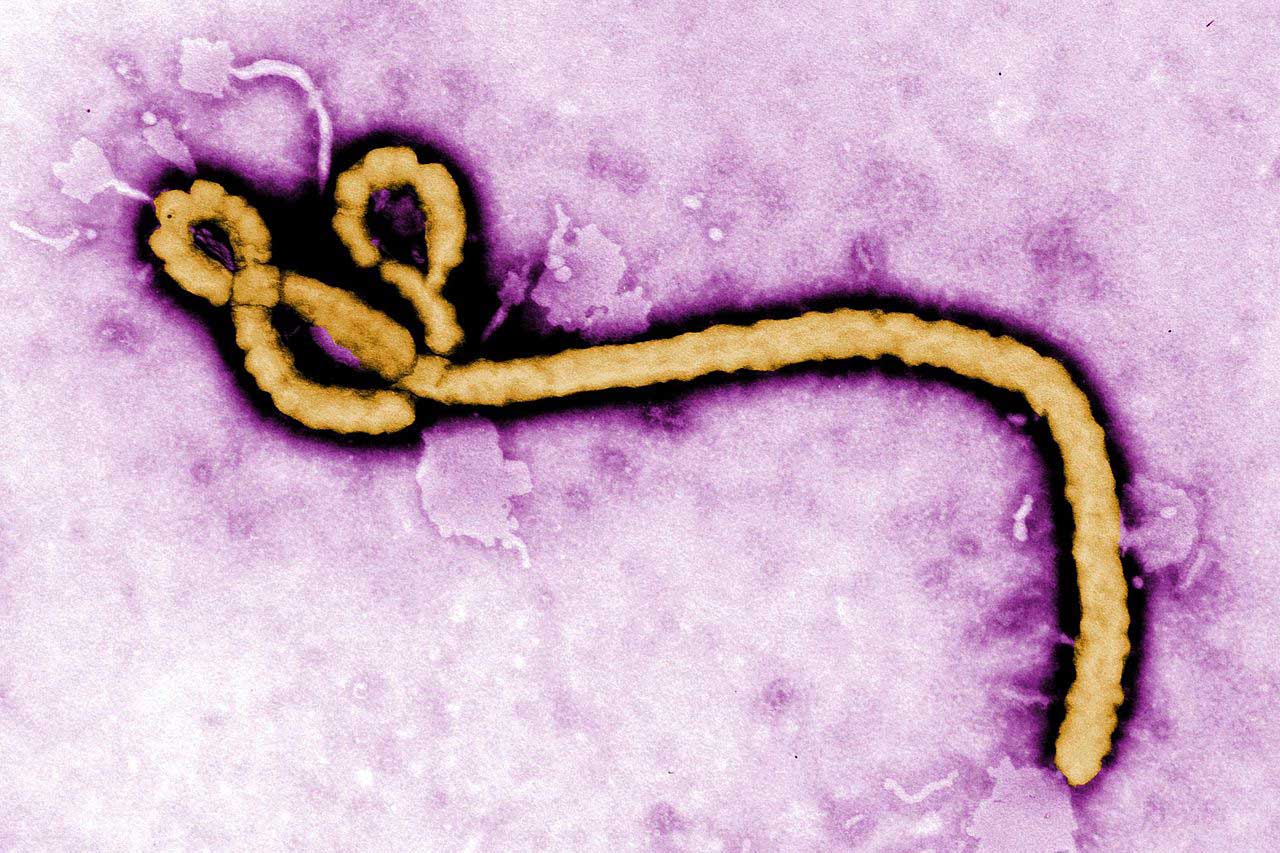
Ebola virus: a brief history
Ebola virus disease was first recognised in two distinct outbreaks in the Ebola River Valley of the Democratic Republic of Congo (formerly Zaire) and the Sudan in 1976. Caused by a relatively simple “filovirus”, a thin, looping innocuous-looking filament of genetic material and proteins, it is spread from person to person through direct contact with infected body fluids. Infection usually results in haemorrhagic fever, which is highly lethal – during early outbreaks, around 90% of people died.
Because Ebola killed so quickly, people infected with the virus often died before passing it to others. That confined its spread to small outbreaks in remote and relatively sparsely populated rural regions of Africa, and for decades after its discovery it remained a relatively low-impact disease. Between its first documented appearance in 1976 and the 2014–15 epidemic, the Ebola virus infected about 2,400 people and killed fewer than 1,600.

Ebola virus disease was first recognised in two distinct outbreaks in the Ebola River Valley of the Democratic Republic of Congo (formerly Zaire) and the Sudan in 1976. Caused by a relatively simple “filovirus”, a thin, looping innocuous-looking filament of genetic material and proteins, it is spread from person to person through direct contact with infected body fluids. Infection usually results in haemorrhagic fever, which is highly lethal – during early outbreaks, around 90% of people died.
Because Ebola killed so quickly, people infected with the virus often died before passing it to others. That confined its spread to small outbreaks in remote and relatively sparsely populated rural regions of Africa, and for decades after its discovery it remained a relatively low-impact disease. Between its first documented appearance in 1976 and the 2014–15 epidemic, the Ebola virus infected about 2,400 people and killed fewer than 1,600.
No commercial market for Ebola vaccines
During this time, scientists researched how to protect against Ebola – a drive mainly fuelled by fears that Ebola could be turned into a bioweapon. Following the terrorist attacks of September 11, 2001, on New York City’s World Trade Center and the Pentagon, the United States of America (USA) dramatically increased biodefence funding. Significant funds were spent developing anti-Ebola vaccines and drugs that could be deployed if the virus was weaponised and intentionally released.
By 2014, 10 or so Ebola vaccines and treatments were in various stages of research, development and clinical testing. The USA was not alone in funding biodefence research related to Ebola. Canada’s Department of National Defence invested US$ 7 million in developing an Ebola vaccine at its National Microbiology Laboratory.
But no commercial market for Ebola vaccines existed. There was no imperative to create a safe, licensed Ebola vaccine that could be stockpiled and deployed to prevent a large-scale natural outbreak of the disease.
During this time, scientists researched how to protect against Ebola – a drive mainly fuelled by fears that Ebola could be turned into a bioweapon. Following the terrorist attacks of September 11, 2001, on New York City’s World Trade Center and the Pentagon, the United States of America (USA) dramatically increased biodefence funding. Significant funds were spent developing anti-Ebola vaccines and drugs that could be deployed if the virus was weaponised and intentionally released.
By 2014, 10 or so Ebola vaccines and treatments were in various stages of research, development and clinical testing. The USA was not alone in funding biodefence research related to Ebola. Canada’s Department of National Defence invested US$ 7 million in developing an Ebola vaccine at its National Microbiology Laboratory.
But no commercial market for Ebola vaccines existed. There was no imperative to create a safe, licensed Ebola vaccine that could be stockpiled and deployed to prevent a large-scale natural outbreak of the disease.
Outbreak starts
Yet on 26 December 2013, such an outbreak began. An 18-month-old boy fell ill in Meliandou, a small village of just 31 houses nestling in the forested Gueckedou district of Guinea. Two days later he died. By the second week of January 2014, several members of the boy’s immediate family had developed a similar illness followed by rapid death. The same fate befell several midwives, traditional healers and staff who had treated the family at the local hospital. On 22 March 2014, a laboratory in France confirmed that the disease was caused by the Zaire strain of Ebola.
Health officials had few tools to prevent its spread. WHO mobilised its Global Outbreak Alert and Response Network, and Médecins Sans Frontières sent field workers to the affected countries. As the virus spread to Sierra Leone, WHO created an emergency response team. But despite growing evidence of the outbreak’s uncontrolled spread, a public health emergency of internal concern was not declared until 8 August, 2014 – 138 days after Ebola was first detected.
That same month, Canada’s federal government donated the vaccine it had researched for biodefence purposes for use in Africa, with the Public Health Agency of Canada licensing its manufacture to NewLink Genetics and Merck.
Yet on 26 December 2013, such an outbreak began. An 18-month-old boy fell ill in Meliandou, a small village of just 31 houses nestling in the forested Gueckedou district of Guinea. Two days later he died. By the second week of January 2014, several members of the boy’s immediate family had developed a similar illness followed by rapid death. The same fate befell several midwives, traditional healers and staff who had treated the family at the local hospital. On 22 March 2014, a laboratory in France confirmed that the disease was caused by the Zaire strain of Ebola.
Health officials had few tools to prevent its spread. WHO mobilised its Global Outbreak Alert and Response Network, and Médecins Sans Frontières sent field workers to the affected countries. As the virus spread to Sierra Leone, WHO created an emergency response team. But despite growing evidence of the outbreak’s uncontrolled spread, a public health emergency of internal concern was not declared until 8 August, 2014 – 138 days after Ebola was first detected.
That same month, Canada’s federal government donated the vaccine it had researched for biodefence purposes for use in Africa, with the Public Health Agency of Canada licensing its manufacture to NewLink Genetics and Merck.
Gavi model in action:
working with manufacturers
In the final days of 2014, the Gavi Board sent a clear signal to manufacturers by committing up to US$ 300 million for Ebola vaccine procurement. This support helped encourage health bodies and manufacturers to invest in the accelerated development of candidate vaccines and begin advanced trials.
In Liberia, the US National Institutes of Health and Liberia Ministry of Health began a randomised, double-blind trial of two experimental vaccines. The first was developed by scientists at GlaxoSmithKline (GSK). The second was the vaccine licensed by Canada to Merck, known as rVSV-ZEBOV.
working with manufacturers
In the final days of 2014, the Gavi Board sent a clear signal to manufacturers by committing up to US$ 300 million for Ebola vaccine procurement. This support helped encourage health bodies and manufacturers to invest in the accelerated development of candidate vaccines and begin advanced trials.
In Liberia, the US National Institutes of Health and Liberia Ministry of Health began a randomised, double-blind trial of two experimental vaccines. The first was developed by scientists at GlaxoSmithKline (GSK). The second was the vaccine licensed by Canada to Merck, known as rVSV-ZEBOV.
Emmanuel: first in line
Emmanuel Lasanah was the first of 1,500 adults who visited Redemption Hospital in Monrovia to receive an inoculation of either vaccine, or a placebo. GSK and Merck provided the vaccines, both of which elicited an immune response that may protect against the Ebola virus. However, the epidemic ended before the clinical trials could be completed.
In 2015, Merck’s experimental vaccine was separately tested in Guinea, where health officials implemented the “Ebola, ça suffit” (“Ebola, that’s enough”) vaccination trial. Almost 12,000 people who had come into contact with someone who had showed symptoms of the disease were vaccinated either immediately or after 21 days.

Emmanuel Lasanah was the first of 1,500 adults who visited Redemption Hospital in Monrovia to receive an inoculation of either vaccine, or a placebo. GSK and Merck provided the vaccines, both of which elicited an immune response that may protect against the Ebola virus. However, the epidemic ended before the clinical trials could be completed.
In 2015, Merck’s experimental vaccine was separately tested in Guinea, where health officials implemented the “Ebola, ça suffit” (“Ebola, that’s enough”) vaccination trial. Almost 12,000 people who had come into contact with someone who had showed symptoms of the disease were vaccinated either immediately or after 21 days.
Guinea was declared Ebola-free on 29 December, 2015.
The trial ended on 20 January the following year, after the final participants had completed their 84-day follow-up. The vaccine proved to be well tolerated in adults. Most importantly, it demonstrated a vaccine efficacy of 100%, meaning that it protected everyone who received it.
But the outbreak was not quite over yet. In March 2016, a flare-up of Ebola virus disease was reported, with two new cases identified in the Nzérékoré prefecture of Guinea. In response, health officials conducted another emergency vaccination programme with the rVSV-ZEBOV vaccine.
Vaccination teams and frozen vaccine doses were flown to the outbreak settings. Within 10 days of the first confirmed case of Ebola, people who may have come into contact with the disease were inoculated. By late April, more than 1,500 people were vaccinated in Guinea, including 307 front-line workers.
These vaccination programmes marked the first time an Ebola vaccine was used in an outbreak setting. Few vaccine-related adverse events were reported, and no secondary cases of Ebola virus disease occurred among the vaccinees.
Gavi model in action: Ebola vaccine stockpile
Sadly, this is not the end of the Ebola story. In 2017, the disease was detected again – this time in the Democratic Republic of the Congo (DRC) in Central Africa. This was identified as the Zaire strain of Ebola.
This time, however, 300,000 doses of Merck’s vaccine, which targets this specific strain of the disease, were available to prevent the disease spreading. This was thanks to Gavi’s Advance Purchase Commitment with Merck, announced in January 2016. Gavi committed an initial US$ 5 million towards the procurement of a vaccine once it is licensed, prequalified and recommended by WHO. As part of this commitment Merck agreed to create and store a stockpile of 300,000 doses of the investigational vaccine, available in the case of an Ebola outbreak pre-licensure.
Work is still ongoing to get an Ebola vaccine WHO-prequalified, deeming it effective, safe, and suitable for general stockpiling and use in emergency. The China FDA announced in October 2017 that it had approved an Ebola vaccine co-developed by its Bioengineering Institute of the Chinese Academy of Military Medical Sciences and private drugmaker Tianjin CanSino Biotechnology Inc. A licence for and WHO prequalification of Merck’s rVSV-ZEBOV vaccine is expected in 2019/2020.

Sadly, this is not the end of the Ebola story. In 2017, the disease was detected again – this time in the Democratic Republic of the Congo (DRC) in Central Africa. This was identified as the Zaire strain of Ebola.
This time, however, 300,000 doses of Merck’s vaccine, which targets this specific strain of the disease, were available to prevent the disease spreading. This was thanks to Gavi’s Advance Purchase Commitment with Merck, announced in January 2016. Gavi committed an initial US$ 5 million towards the procurement of a vaccine once it is licensed, prequalified and recommended by WHO. As part of this commitment Merck agreed to create and store a stockpile of 300,000 doses of the investigational vaccine, available in the case of an Ebola outbreak pre-licensure.
Work is still ongoing to get an Ebola vaccine WHO-prequalified, deeming it effective, safe, and suitable for general stockpiling and use in emergency. The China FDA announced in October 2017 that it had approved an Ebola vaccine co-developed by its Bioengineering Institute of the Chinese Academy of Military Medical Sciences and private drugmaker Tianjin CanSino Biotechnology Inc. A licence for and WHO prequalification of Merck’s rVSV-ZEBOV vaccine is expected in 2019/2020.
Rebuilding health systems
Developing new vaccines to prevent devastating outbreaks is vital, but it is not sufficient.
Vaccines do not deliver themselves: they require the right equipment, trained staff and sufficiently strong health systems to safely reach the people who need them.
The Ebola crisis wreaked havoc on the health systems of Guinea, Liberia and Sierra Leone. Hundreds of trained health workers died and many were forced to abandon their posts as the epidemic tightened its grip, leading to severe staff shortages. False rumours circulated throughout the region suggesting that childhood vaccines, such as those that protect against measles, were themselves linked to Ebola.
Immunisation and other essential health services were dealt a severe blow.

Developing new vaccines to prevent devastating outbreaks is vital, but it is not sufficient.
Vaccines do not deliver themselves: they require the right equipment, trained staff and sufficiently strong health systems to safely reach the people who need them.
The Ebola crisis wreaked havoc on the health systems of Guinea, Liberia and Sierra Leone. Hundreds of trained health workers died and many were forced to abandon their posts as the epidemic tightened its grip, leading to severe staff shortages. False rumours circulated throughout the region suggesting that childhood vaccines, such as those that protect against measles, were themselves linked to Ebola.
Immunisation and other essential health services were dealt a severe blow.
Across the three countries, vaccination coverage dropped by approximately 50%. Post-Ebola, Gavi allocated up to US$ 45 million to support the recovery of health and immunisation systems , including disbursing funds to support the implementation of Guinea, Liberia and Sierra Leone’s Expanded Programme on Immunization (EPI) recovery plans in the 2015–2016 period.
Thanks partially to this support, in the wake of the Ebola outbreak there were no large-scale epidemics of vaccine-preventable diseases. Liberia, for example, used some of its funds to revitalise outreach services for routine immunisation management in 15 counties. It also developed communication materials to rebuild trust and confidence in health services.
To support the continued recovery progress in all three countries, the Vaccine Alliance doubled the ceiling for its health system strengthening grants to a total of US$ 61 million for the five-year period following the outbreak.
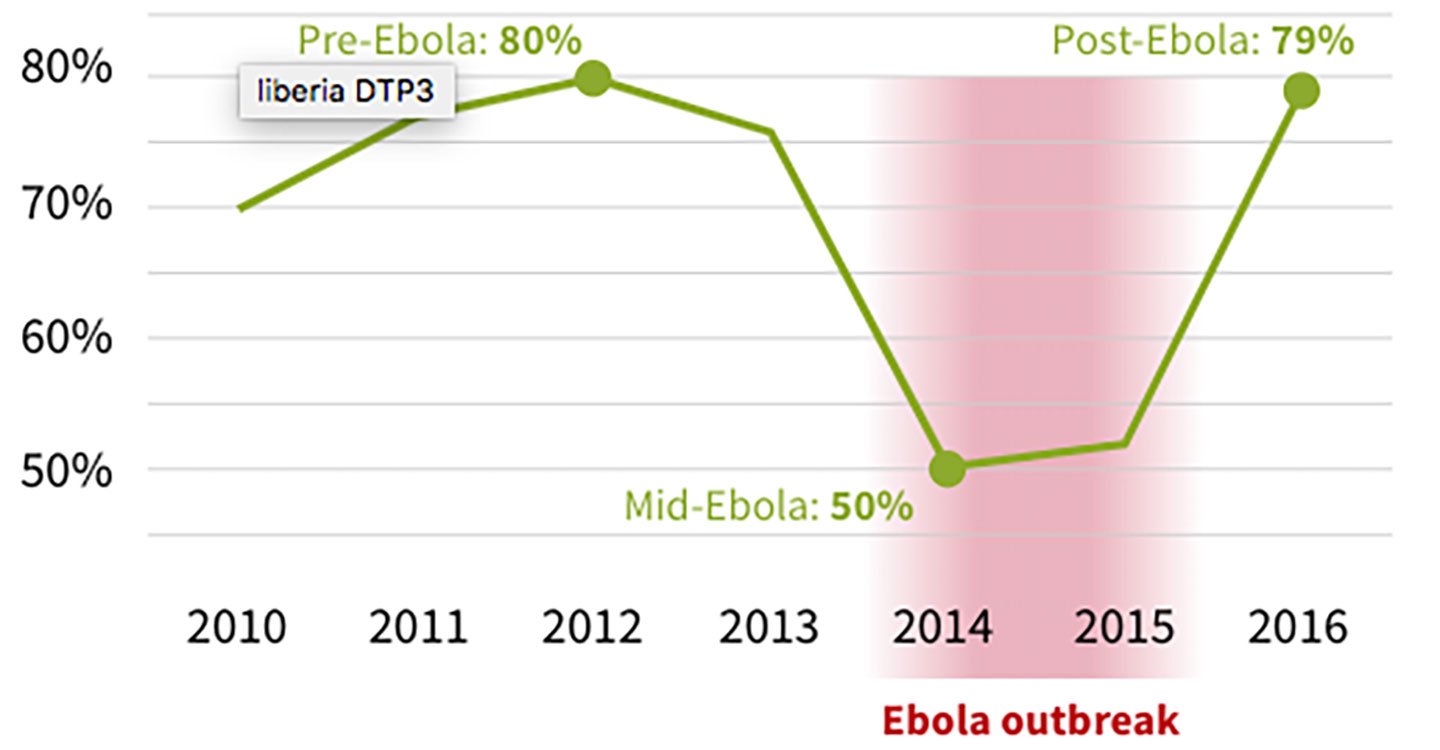
Across the three countries, vaccination coverage dropped by approximately 50%. Post-Ebola, Gavi allocated up to US$ 45 million to support the recovery of health and immunisation systems , including disbursing funds to support the implementation of Guinea, Liberia and Sierra Leone’s Expanded Programme on Immunization (EPI) recovery plans in the 2015–2016 period.
Thanks partially to this support, in the wake of the Ebola outbreak there were no large-scale epidemics of vaccine-preventable diseases. Liberia, for example, used some of its funds to revitalise outreach services for routine immunisation management in 15 counties. It also developed communication materials to rebuild trust and confidence in health services.
To support the continued recovery progress in all three countries, the Vaccine Alliance doubled the ceiling for its health system strengthening grants to a total of US$ 61 million for the five-year period following the outbreak.
Gavi model in action: innovating the cold chain
The experimental Ebola vaccine needs to be stored at temperatures below -70° C to stay effective. Vials of the vaccine exposed to temperatures above +25° C, which are common in Africa, quickly become unstable.
During the outbreak in West Africa, health workers transported the vaccine in an innovative, portable cold box known as Arktek, which works like a regular coffee thermos. Developed by Global Good, a collaboration between the Bill & Melinda Gates Foundation and the innovation lab Intellectual Ventures, Arktek has an inner bottle containing the vaccines and ice, separated from the outer bottle via a vacuum space. The new cooler is designed to maintain temperatures as low as -80° C for up to two months without power.
Gavi helps developing countries buy the Arktek and other essential equipment for vaccine delivery and storage through its cold chain equipment optimisation platform. From 2016 to 2017, the Vaccine Alliance committed an initial US$ 50 million to modernise cold chain equipment in the world’s poorest countries. This investment was subsequently increased five-fold to match anticipated demand over the next five-year period.

The experimental Ebola vaccine needs to be stored at temperatures below -70° C to stay effective. Vials of the vaccine exposed to temperatures above +25° C, which are common in Africa, quickly become unstable.
During the outbreak in West Africa, health workers transported the vaccine in an innovative, portable cold box known as Arktek, which works like a regular coffee thermos. Developed by Global Good, a collaboration between the Bill & Melinda Gates Foundation and the innovation lab Intellectual Ventures, Arktek has an inner bottle containing the vaccines and ice, separated from the outer bottle via a vacuum space. The new cooler is designed to maintain temperatures as low as -80° C for up to two months without power.
Gavi helps developing countries buy the Arktek and other essential equipment for vaccine delivery and storage through its cold chain equipment optimisation platform. From 2016 to 2017, the Vaccine Alliance committed an initial US$ 50 million to modernise cold chain equipment in the world’s poorest countries. This investment was subsequently increased five-fold to match anticipated demand over the next five-year period.
Ebola's legacy
The world may yet face another major outbreak of Ebola. Recognising that prevention is always better than cure, the Vaccine Alliance is working with a range of partners to try to put the right safeguards in place.
In addition to helping to encourage the development and future licensure and prequalification of the Ebola vaccine, the Vaccine Alliance will fund stockpiles of the vaccine once it is available. It also supports the right cold chain equipment to safely store and transport it, and strengthens health systems so they are better able to prevent, identify and react to outbreaks.
But the threat goes wider. With Ebola’s deadly legacy fresh in everyone’s memory, in December 2015 WHO brought together scientific experts to draw up a list of 11 diseases of epidemic potential for which few licensed drugs or vaccines exist. The list included Ebola and other haemorrhagic fevers such as Marburg and Lassa fever, and Zika, a viral disease thought to have caused deformities in new-born children in Brazil and other countries.

The world may yet face another major outbreak of Ebola. Recognising that prevention is always better than cure, the Vaccine Alliance is working with a range of partners to try to put the right safeguards in place.
In addition to helping to encourage the development and future licensure and prequalification of the Ebola vaccine, the Vaccine Alliance will fund stockpiles of the vaccine once it is available. It also supports the right cold chain equipment to safely store and transport it, and strengthens health systems so they are better able to prevent, identify and react to outbreaks.
But the threat goes wider. With Ebola’s deadly legacy fresh in everyone’s memory, in December 2015 WHO brought together scientific experts to draw up a list of 11 diseases of epidemic potential for which few licensed drugs or vaccines exist. The list included Ebola and other haemorrhagic fevers such as Marburg and Lassa fever, and Zika, a viral disease thought to have caused deformities in new-born children in Brazil and other countries.
Collaboration with partners
Gavi has already played an instrumental role in making the experimental Ebola vaccine available. To help accelerate the development of vaccines that protect against the other 10 diseases on WHO’s list, Gavi has been involved in the establishment of the Coalition for Epidemic Preparedness and Innovation (CEPI). Founding partners include the Governments of India and Norway, the Bill & Melinda Gates Foundation, the Wellcome Trust and the World Economic Forum.
CEPI plans to create a sustainable model for the development of these vaccines, moving beyond the ad-hoc partnerships and goodwill of governments, agencies and companies that defined the reaction to the Ebola outbreak.
CEPI will initially concentrate on vaccines for known epidemic threats. It will also build capabilities and partnerships that could rapidly be used against new epidemic diseases we are not aware of today, as and when they emerge. If successful, this model could eventually be extended to cover endemic diseases and other medical interventions.
In the future, that might spare people such as Emmanuel Lasanah, and hundreds of thousands of others, the devastation and loss of life caused by unexpected disease outbreaks.

Gavi has already played an instrumental role in making the experimental Ebola vaccine available. To help accelerate the development of vaccines that protect against the other 10 diseases on WHO’s list, Gavi has been involved in the establishment of the Coalition for Epidemic Preparedness and Innovation (CEPI). Founding partners include the Governments of India and Norway, the Bill & Melinda Gates Foundation, the Wellcome Trust and the World Economic Forum.
CEPI plans to create a sustainable model for the development of these vaccines, moving beyond the ad-hoc partnerships and goodwill of governments, agencies and companies that defined the reaction to the Ebola outbreak.
CEPI will initially concentrate on vaccines for known epidemic threats. It will also build capabilities and partnerships that could rapidly be used against new epidemic diseases we are not aware of today, as and when they emerge. If successful, this model could eventually be extended to cover endemic diseases and other medical interventions.
In the future, that might spare people such as Emmanuel Lasanah, and hundreds of thousands of others, the devastation and loss of life caused by unexpected disease outbreaks.