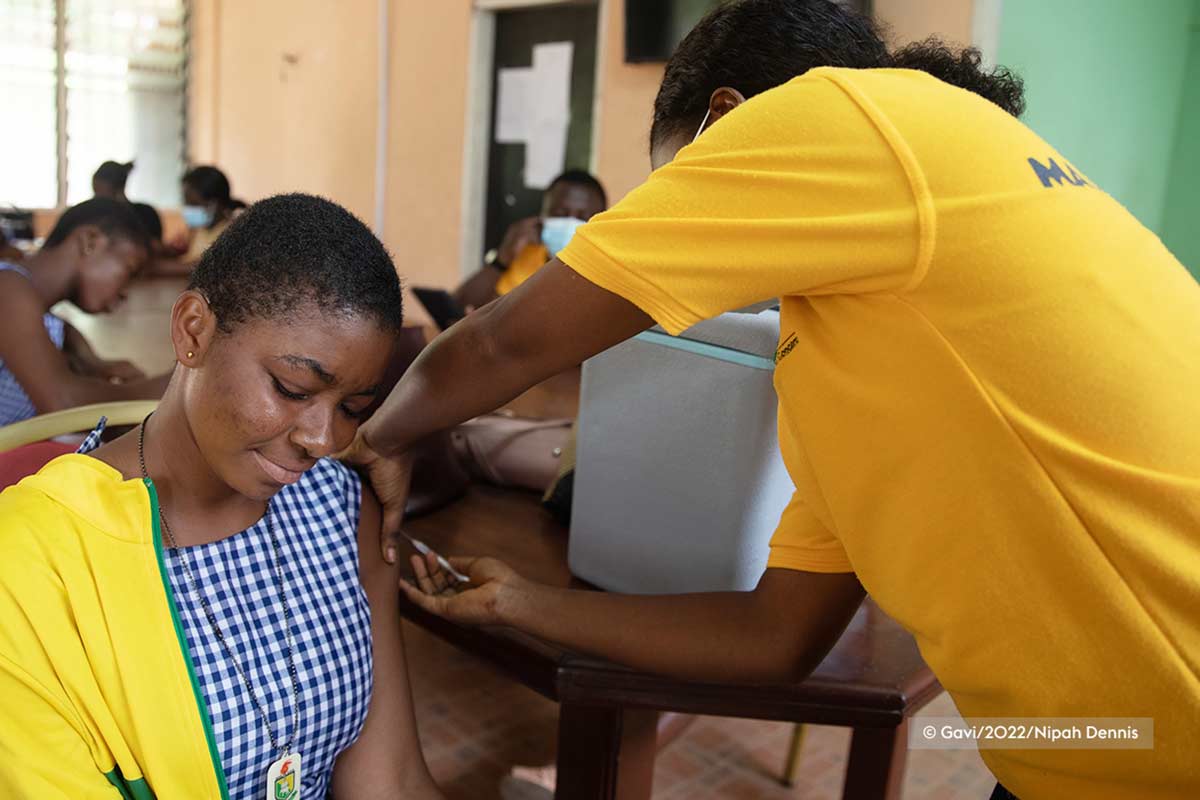Clinical trials in remote rainforests: how vital Ebola research in DRC enhances local expertise and infrastructure
In the heart of the equatorial rainforest lies the remote city of Boende, Democratic Republic of Congo. Nestled in 178 million hectares of rich biodiversity, it is home to thousands of people. This area was hit hard by a deadly Ebola outbreak in 2014 that killed 49 people. Conversations about Ebola are still painful for many in the community, where the threat of future outbreaks persists.
- 30 August 2023
- 8 min read
- by CEPI

Professor Hypolite Muhindo-Mavoko and his team from the University of Kinshasa conduct vital research from this isolated part of the world. Having constructed a clinical trial site for an Ebola vaccine trial called EBL2007 (conducted by the EBOVAC3 Consortium) in 2019, the facilities are now used for several ongoing projects. Money invested in these projects not only generates much needed clinical trial data, it also supports local health infrastructure and services, and builds clinical research capacity by providing training and employment for staff in the region.
Working with the European Union's IMI Programme, CEPI provided funding to support the Ebola vaccine trial in Boende as part of its work to generate additional clinical data which could expand access to Ebola vaccines. CEPI also funds a consortium of African research institutions evaluating COVID-19 vaccines in people living with HIV, which Professor Mavoko's team is part of.
Professor Mavoko met with CEPI to tell us more about the project and the impact this work is having on the community.
Tell us about yourself and your work
I’m Hypolite Muhindo-Mavoko, Professor in the Department of Tropical Medicine at the University of Kinshasa and I work in the field of tropical infectious diseases. The Department was founded in 2006. It was created to train people involved in the control of tropical diseases, which are prevalent in DRC. We also conduct research that supports policymakers, so evidence-based policies are produced locally.

Credit: CEPI
We are involved in a project known as EBOVAC3 via colleagues at the University of Antwerp who we have a long-standing relationship with. The project aims to increase preparedness for Ebola outbreaks in DRC, which has experienced the most frequent Ebola outbreaks. As part of this project, we've vaccinated healthcare workers and frontliners in the Tshuapa province in DRC, a province that faced an Ebola outbreak in 2014. Health facilities play a major amplification role in disease transmission, so the idea was to vaccinate a population that can easily come in to contact with the disease.
Ebola virus disease is a life-threatening disease that can be devastating. Fatality rates can reach 90%. In an outbreak situation, the idea is to contain the outbreak, and absolutely avoid it spreading. It's important to have weapons to fight it that are evidence-based and proven to be effective against the virus.
I've also been working as part of a CEPI-funded consortium project led by Victoria Biomedical Research Institute (VIBRI), which is undertaking COVID-19 mix and match vaccine trials across DRC, Rwanda, and Kenya.
Tell us more about the EBOVAC3 study
As part of the EBOVAC3 project, the EBL2007 trial was conducted in DRC. This was a randomised Phase 2 trial to investigate the safety and immunogenicity of a heterologous vaccine regimen comprised of two vaccines, Ad26.ZEBOV and MVA-BN-Filo, administered 56 days apart. We enrolled 700 healthcare workers and frontline workers from the district of Boende. The randomisation was done at enrolment: one group received a booster dose (Ad26.ZEBOV) after one year, the other group received a booster dose after two years. We monitored for any serious adverse events up to six months after each vaccination. The results, which are currently under peer review, will also inform us about the immunogenicity and safety of the vaccine and identify the appropriate time to administer the booster dose. The EBOVAC3 study is also gathering safety and immunogenicity data on the Ad26.ZEBOV and MVA-BN-Filo prime-boost Ebola vaccine regimen in infants in Sierra Leone and Guinea.

CEPI provided funding for the EBOVAC3 to support project monitoring, which was in place to ensure trial procedures were completed correctly. CEPI has been very supportive and I'm grateful for the Coalition's efforts to help strengthen the capacity of our team in the field.
How was the EBOVAC3 project perceived by the community? What impact has it had locally?
The EBOVAC3 project was well perceived in the community, which still has bad memories of the Ebola outbreak in 2014. Colleagues assessing perception of trials in the community visited areas including the village where the first cases of the 2014 outbreak occurred. When they asked questions about Ebola, some people started crying and saying they never wished to hear about Ebola again.
Some community members asked us why participant selection for the trial was limited to healthcare workers, because many wanted to receive the vaccination. If protection is available, they are willing to receive it. That is why I believe there is still a lot that needs to be done in that province in terms of protection. But also, in terms of exploring research questions around Ebola, mpox, and many other emerging infectious diseases.
Have you read?
The sharing of knowledge between teams has been a great experience and enabled us to build capacity in Boende. For instance, as part of the EBOVAC3 project, half the staff came from Kinshasa and worked with the local team, who taught them to conduct clinical research in compliance with Good Clinical Practice principles. Our department is now conducting a malaria trial on behalf of the National Malaria Control programme and a COVID-19 mix and match trial funded by CEPI. For this mix and match trial, we now have more team members who have been recruited locally in Boende because of the capacity that was built through the EBOVAC3 study.
In Boende, medical doctors weren't previously trained in resuscitation and intensive care. So at the beginning of the EBOVAC3 project, two medical doctors from the local hospital were taken to Kinshasa for a month's training. They received tuition in resuscitation and they were able to take that knowledge back and share it locally.
In a province larger than 20,000 square kilometers, our group brought the first machines capable of running biochemistry blood analysis. Our lab is based in the General Hospital, so if the population needs biochemistry analysis checks for liver or kidney function, for example, our team helps free of charge.
We also have a project pharmacy where common medicines are available. Availability of this resource proved crucial during a shortage of anti-malarial medications, which are usually provided through the National Malaria Control Programme. In response to this shortage, patients were referred to our pharmacy, where we were able to fulfil their medication needs from our stocks.
We have three large generators running 24 hours, which gives us access to electricity and provides light to the hospital. We use generators 24/7 in Boende to power our lab and maintain our cold-chain. We have also built a water supply in the hospital, which is used by other wards.
Our work is also having a global impact. We share data from our research with the global scientific community, which also helps to increase awareness of the type of research activities that are being conducted in Boende. I think it's good for the city and the whole province.
What kind of challenges have you faced in conducting the EBOVAC3 trial?
We found the administrative procedures challenging. There was a huge Ebola outbreak in eastern Congo at the beginning of our trial. Initially, only one type of vaccine (rVSV-ZEBOV) was allowed to be deployed, which was different from the one intended for our study. This threatened the whole trial. Eventually, we got approval to use the Ad26.ZEBOV/MVA-BN-Filo vaccine, which was being assessed as part of EBOVAC3.

Boende is a remote town located in the equatorial forest. You can't access it by road—there is no road at all from Kinshasa, the capital city. The only means of transport are small aircrafts. There was no internet, no electricity, or trained staff available, meaning we had to transport supplies from Kinshasa to construct the site and hire and train medical staff locally. It was crucial to meticulously plan logistics for every single item that we needed because supply flights only came every seven days. Items that were too large for an aircraft must be sent by boat, which can take two or more weeks to arrive.
We wanted to contribute to the local economy, so we planned to source project equipment locally wherever possible. One day during the project, there was no paper left in any of the shops in the city because we had bought it all. In the end we had to ship more paper from Kinshasa.
The language barrier is also a challenge. While English is the language commonly used in the global scientific community, the main language in DRC is French. Day-to-day, my team make huge efforts to interact in English, so we can be active and competitive in global research networks. We published our experience of setting up the EBOVAC3 trial so that other researchers who are planning work in similar environments can learn how to overcome those challenges.
In the context of this conversation, is there anything else you'd like to address?
Historically, when competing for research grants, institutions in the Global South stand little chance against institutions from the North. I think it's important to change that trend and give more responsibility to researchers and institutions in the Global South so that we can develop not only the scientific skills but also the technical and administrative skills.
Strengthening technical capacities is also crucial. Up to now, we are shipping lab samples abroad for analysis and experience very long delays receiving results. If we had the logistics and facilities in place to process the samples locally, I think it would be beneficial.
If we are given the opportunity to conduct more studies, to set up clinical trial sites all over the province, I think that would be very interesting. There are opportunities not only for Ebola research but other viral diseases like measles and mPox, which are also endemic in the province. Those are the fields which we would be grateful to contribute to and help find solutions.
I'd like to express my thanks for the opportunity to have this discussion. It's vital for me and my institution to interact with people across the world - it contributes to making us strong.
The interview has been edited for brevity and clarity.
Written by
Rowena Madar
Website
This article was originally published by CEPI.