How new vaccines could revolutionise our relationship with tuberculosis
Hopes are high for a new TB vaccine now in the last stages of development, but other candidates aren’t far behind it.
- 13 May 2025
- 6 min read
- by Linda Geddes
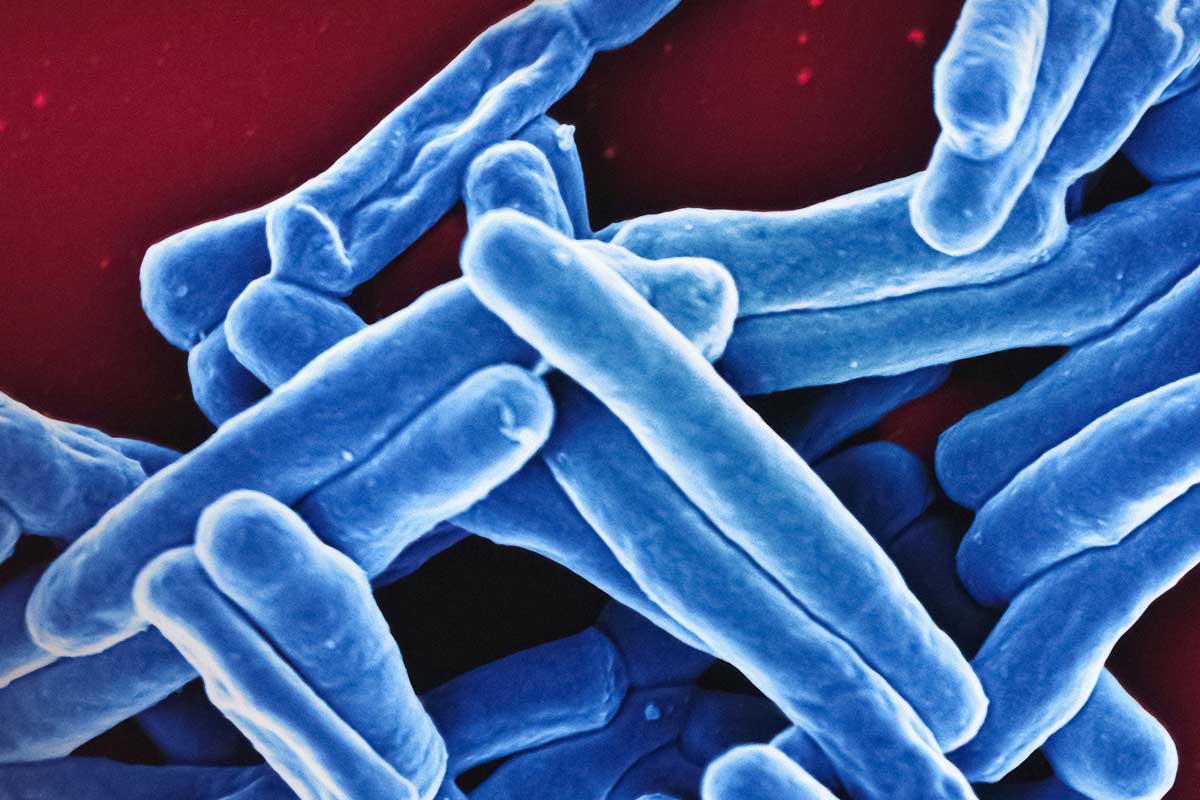
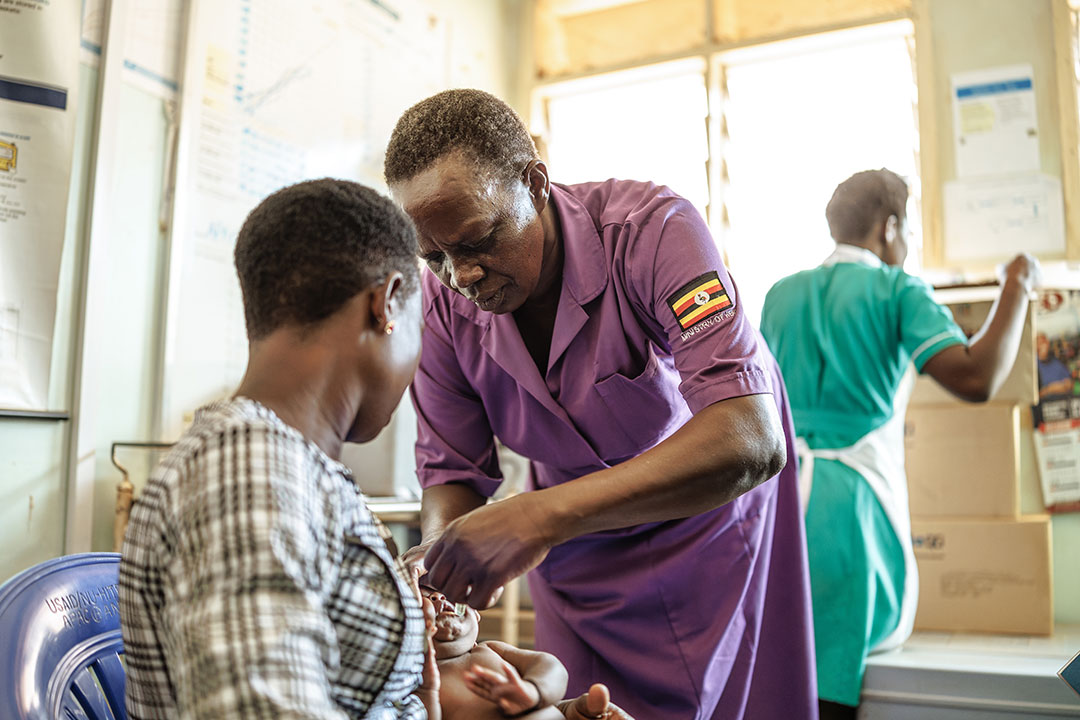
Tuberculosis (TB) has scourged humanity for millennia and remains the deadliest infectious disease worldwide. Although a cheap and partially effective vaccine has long been available, new ones are urgently needed.
News that a potential breakthrough vaccine – one that many hope could be the first new TB vaccine for a century – has reached its clinical trial recruitment target ahead of time is understandably stirring optimism. It’s not alone, either: several other contenders are close on its heels.
So what might these next-generation vaccines mean for the way we live with, and defend against, TB in the years to come?
Ancient killer
Every three minutes, tuberculosis silently claims another three lives, making it the world’s biggest infectious killer.
In 2021 alone, 10.6 million people were diagnosed with TB and 1.6 million died. Even though the disease is curable with antibiotics, millions still slip through the cracks, undiagnosed, untreated or facing forms of TB that no longer respond to standard antibiotics.
The only vaccine currently in widespread use, BCG, offers moderate protection against severe forms of TB in infants and young children, including TB disease of the brain (meningitis), and miliary TB – where the bacterium spreads through the bloodstream and affects multiple organs.
But for adolescents and adults, who are responsible for the lion’s share of transmission, its effectiveness wanes. The BCG vaccine also doesn’t prevent the transmission of pulmonary TB, a type that affects the lungs. New vaccines could be a game-changer.
“There is hope that a new tuberculosis vaccine targeting adolescents and adults could bring the numbers significantly down,” says Marta Tufet Bayona, head of policy at Gavi, the Vaccine Alliance.
When Gavi recently assessed the potential impact that such vaccines could have in the countries it supports, it estimated that delivering a TB vaccine through routine immunisation of 15-year-olds could avert 201,000–230,000 deaths and 64–73 million years of healthy lives lost between 2026 and 2040.
Researchers at the Harvard T.H. Chan School of Public Health in Boston, US, have also suggested that an effective TB vaccine for adolescents and adults could produce up to US$ 474 billion in economic benefits by 2050.
Vaccine frontrunner
While there are at least 20 TB vaccine candidates for adults and adolescents in the clinical pipeline, the current frontrunner is the M72/AS01E (M72) vaccine.
It is built around a fusion protein called M72, which combines fragments from two proteins found in Mycobacterium tuberculosis, the bacterium that causes TB. To boost the body’s immune response, the vaccine also contains an adjuvant called AS01E, which is also used to boost immune responses to the RTS,S malaria vaccine, respiratory syncytial virus (RSV) vaccine for adults and the Shingrix shingles vaccine.
The results of its phase 2b trial, involving more than 3,500 HIV-negative adults in countries with high TB burdens, were cautiously encouraging.
In these adults, who were already thought to have been infected with the bacterium (TB bacteria can remain dormant for many years), the vaccine halved the risk of developing active TB over the next three years compared to placebo, with an estimated efficacy of 50%.
A Phase 3 trial of the M72 vaccine in adolescents and adults began in March 2024, with all 20,000 participants now having been recruited across 54 sites in South Africa, Kenya, Malawi, Zambia and Indonesia.
Although researchers had anticipated it could take up to two years to enrol the targeted number of participants, the study has reached its target ahead of schedule, marking an important milestone in the global effort to curb TB.
“There’s still uncertainty on timelines, because the trial depends on participants developing the disease naturally over time, and the rate at which this occurs can vary, but it is really positive that they have managed to recruit at all sites so quickly,” says Tufet Bayona. “It’s important to note that those who do develop the disease during the trial will receive the recommended TB treatment.”
Assuming trials of the M72 vaccine are successful, it could still take several years for the vaccine to become more widely available. “It is estimated that a potential licensure could happen by 2030, but for Gavi to support a vaccination programme, the vaccine would also need to obtain WHO prequalification and a recommendation by WHO’s Strategic Advisory Group of Experts on Immunization (SAGE),” Tufet Bayona says.
Outstanding questions
Even when vaccines show promising Phase 3 trial results, SAGE may request further studies to address any key outstanding questions.
For instance, after publication of Phase 3 results for the RTS,S malaria vaccine in 2015, SAGE sought real-world data on the feasibility of introducing it, uptake of the four-dose schedule, safety, impact on deaths and cost-effectiveness. These pilot studies, launched in 2019, provided essential evidence that informed WHO’s 2021 recommendation for broader use in areas with moderate to high malaria transmission.
In the case of a TB vaccine candidate like M72, which is intended to protect adolescents and adults, there may be questions around the feasibility of introducing it through a routine immunisation programme targeting 15-year-olds, say.
“Unlike infants, adolescents fall outside existing routine vaccination systems. These are healthy kids; they might not necessarily be in school, or coming to clinics on a regular basis, so there are big questions around feasibility,” Tufet Bayona says.
Although Gavi hasn’t committed to financing any TB programmes during its next strategic period, which runs from 2026–2030, its Board has recommended that preparatory work be carried out to assess how a potential new TB vaccine for adults and adolescents could be seamlessly integrated into existing healthcare services, the cost-effectiveness of doing so and the likely demand for such vaccines, if and when they are launched, to help ensure that vaccine supply can keep up with this demand.
Have you read?
Additional candidates
M72 isn’t the only TB vaccine candidate that’s generating excitement. Following close behind it is MTBVAC, a live attenuated vaccine derived from M. tuberculosis, which is currently undergoing Phase 3 trials in newborns across several sub-Saharan African countries, while a Phase 3 trial targeting adolescents and adults is expected to start in India during 2025.
A Phase 3 clinical trial of the VPM1002 tuberculosis vaccine has also commenced in India, targeting adolescents and adults. This candidate is based on a weakened version of the Mycobacterium bovis bacterium used in the existing BCG vaccine.
Various other candidates are also in human clinical trials. All of this is good news for countries bearing the highest burden of tuberculosis, where the approval of new vaccines against this ancient killer couldn’t come a moment too soon.
Ideally, there would be several new vaccines, which could help ensure that demand for them can be met, once they finally are approved. The latest news about the M72 vaccine is a small, but exciting step towards that goal.