How the next wave of vaccines could target human parasites
As countries begin to vaccinate children against malaria, hopes are growing that vaccines against other human parasites could soon be within reach.
- 3 April 2024
- 14 min read
- by Linda Geddes

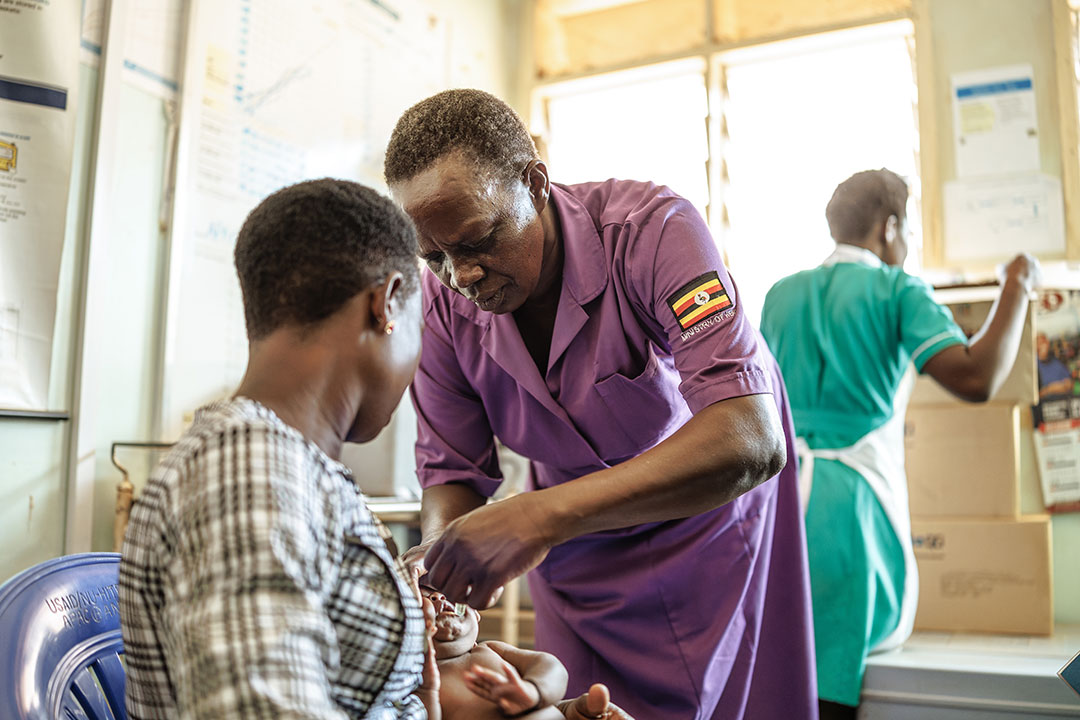
Tafadzwa was warned not to swim in the river. But after a day tending livestock under the scorching Zimbabwean sun, he and his friends disobeyed their parents' orders and plunged into the muddy waters to cool off. He wasn't to know that the river was contaminated with Schistosoma larvae: parasites excreted in human waste that use freshwater snails as a host.
Several weeks later, Tafadzwa began experiencing stomach pain and headaches. Then he started finding blood in his urine. Concerned, his parents took him to a clinic, where the doctor started to ask awkward questions.
"Because of the magnitude of infection – almost 300 million people infected, and a billion people exposed to risk worldwide – the impact of schistosomiasis is huge."
– Dr Miriam Tendler, schistosomiasis researcher, Oswaldo Cruz Foundation, Rio De Janeiro, Brazil.
"The interrogation revealed that I was playing in the river, against my parents' warning not to do so," Tafadzwa said.
He was diagnosed with bilharzia (schistosomiasis), a disease caused by parasitic blood flukes that develop inside freshwater snails, before reinfecting humans if they go swimming, fishing or wash in infected water. Unpleasant as his symptoms were, Tafadzwa was lucky that his infection was detected and treated quickly.
In children, schistosomiasis can cause anaemia and stunt their growth. As they grow older, chronic parasitic infection may affect their ability to work, bear children of their own, or, in severe cases, trigger cancer, liver or kidney failure. In Zimbabwe, schistosomiasis is one of the top ten reasons for people being admitted to hospital.
"Because of the magnitude of infection – almost 300 million people infected, and a billion people exposed to risk worldwide – the impact of schistosomiasis is huge," says Dr Miriam Tendler, a schistosomiasis researcher at the Oswaldo Cruz Foundation in Rio De Janeiro, Brazil. "Particularly for women and children, it affects their quality of life, their ability to go to school or work, and it is the main cause of sterility in young women."
Schistosomiasis isn't the only problematic parasite. A more voracious blood-eating worm, hookworm, is one of the leading causes of anaemia in low- and middle-income countries. Then there's leishmaniasis, a painful and disfiguring infection caused by single-celled parasites called protozoa that are transmitted by the bites of infected sandflies. The most dangerous type, visceral leishmaniasis (kala-azar), is fatal in more than 95% of cases if left untreated and is the second deadliest parasitic disease after malaria.
In 2019 alone, these three parasites alone accounted for 3.3 million years of life lost to ill-health, disability or early death around the world, though their true toll may be even higher.
"Particularly for women and children, it affects their quality of life, their ability to go to school or work, and it is the main cause of sterility in young women."
– Dr Miriam Tendler, schistosomiasis researcher, Oswaldo Cruz Foundation, Brazil.
"Often, we measure the burden of disease based on the longevity of the clinical symptoms. But if one starts to think about a disease that causes major facial scarring, for example, then the impact on quality of life is lifelong – particularly people's mental health," says Prof Paul Kaye at Hull York Medical School in the UK, who has been developing a leishmaniasis vaccine. "If you start to factor in some of those longer-term sequelae into estimates of burden of disease, it jumps up about tenfold from what is currently reported."
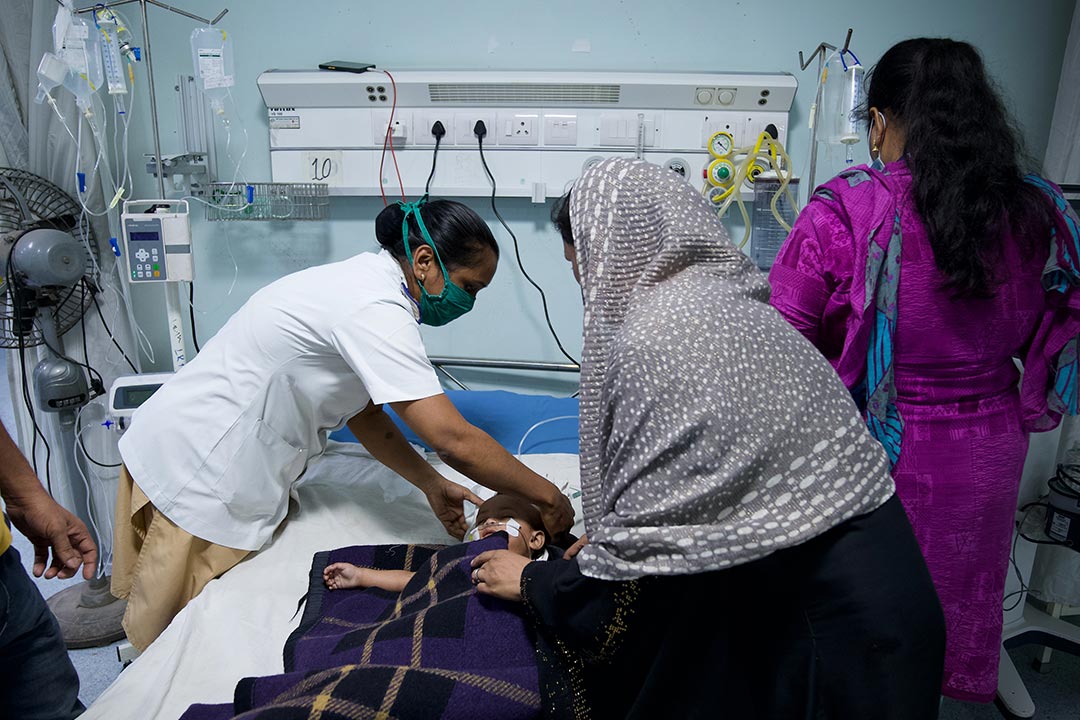
Although parasitic infections can be treated with drugs, as Tafadzwa's was, these do not stop people from being reinfected. Some of them also have unpleasant side-effects and drug resistance is a growing problem. Treatment is unaffordable for some families.
"If you take leishmaniasis, a course of drug treatment would be equivalent to the loss of about six to nine months' worth of income for an individual family in many parts of the world," says Kaye.
For decades, researchers have tried, and failed, to develop vaccines against human parasitic infections. Doing so is far more challenging than vaccinating against bacteria or viruses. Not only are parasites larger and more complex – adult schistosomes are up to 2 cm long – they often have multi-stage life cycles, during which they may change their appearance to confuse the immune system or inhabit different hosts.
Also, while vaccine-induced antibodies may be enough to block or destroy bacteria and viruses, specific T cell-based immune responses are needed to dislodge and prevent parasitic infections, and these are more challenging to generate through vaccination.
"Often, we measure the burden of disease based on the longevity of the clinical symptoms. But if one starts to think about a disease that causes major facial scarring, for example, then the impact on quality of life is lifelong – particularly people’s mental health."
– Prof Paul Kaye, Hull York Medical School, UK
Even so, the approval of vaccines against malaria – the first against a human parasite – and the results of recent clinical trials are fuelling hopes that vaccines against other parasitic diseases may be within reach.
"I could imagine that within the next decade, four or five parasitic vaccines will become available," says Prof Peter Hotez, a vaccine researcher at Baylor College of Medicine and Texas Children's Hospital in the US.
Neglected diseases
Vaccinating against parasites isn't an entirely new concept. "Right back to the early Middle Ages, there was a process used in Iran, called leishmanisation, where women would get samples of leishmaniasis injected into their backsides – because it was an area that wasn't visible – to try and prevent future facial scarring," says Prof John Kelly at the London School of Hygiene and Tropical Medicine, UK, who studies leishmaniasis and other parasitic infections.
Several vaccines have also been licensed that tackle animal parasites including lungworm, barber's pole worm and tapeworm. However, the development of human parasite vaccines has been slower. "Besides the biological challenges, most of these parasitic infections fall into the category of neglected diseases – and that neglect ranges all the way from public awareness through to the ability of health systems to pay for control measures even if they existed, and a lack of incentives for pharma to become engaged in vaccine development," says Kaye.
"Parasitic diseases predominantly affect poor populations living in low- and middle-income countries, and so there is not such a commercially interesting market as there is for other vaccines," Tendler says.
Hookworm is a prime example. It is strongly associated with poverty, and often co-exists with malaria. Both infections also help to keep people poor by destroying or removing their red blood cells, resulting in severe anaemia, which impairs children's school performance and reduces adults' ability to work. These infections are particularly dangerous during pregnancy, because they reduce the oxygen-carrying capacity of blood at a time when more oxygen is needed to support the growth of the baby.
"Right back to the early Middle Ages, there was a process used in Iran, called leishmanisation, where women would get samples of leishmaniasis injected into their backsides – because it was an area that wasn’t visible – to try and prevent future facial scarring."
– Prof John Kelly, London School of Hygiene and Tropical Medicine, UK
"When a woman dies in childbirth in Africa, it is not because she bleeds more, but because she has a low haematocrit [a low number of red blood cells], and that's because she has combined malaria and hookworm – or sometimes malaria, hookworm and schistosomiasis," says Hotez.
He has dreamed of creating a vaccine against hookworm since his days as an MD-PhD student, when he set out to identify the molecular mechanisms hookworms use to feed. This was the 1980s, and his lab at Rockefeller University was just up the street from New York University, where fledgling efforts to develop a malaria vaccine were also underway. Forty years later, the first such vaccines are currently being rolled out in Africa, where they are expected to help save millions of lives.
Have you read?
People usually catch hookworm during contact with soil or crops that are contaminated with human faeces, either because of outdoor defecation, its use as fertiliser or poor sanitation. Once hookworm larvae have burrowed through a person's skin, they are carried to the lungs via their circulatory system, and then crawl up the person's windpipe, causing them to cough up, and then swallow the larvae. These then travel to the gut, where they latch onto the wall of the upper small intestine with their hook-shaped mouths and feed on the person's blood, shedding eggs into their faeces.
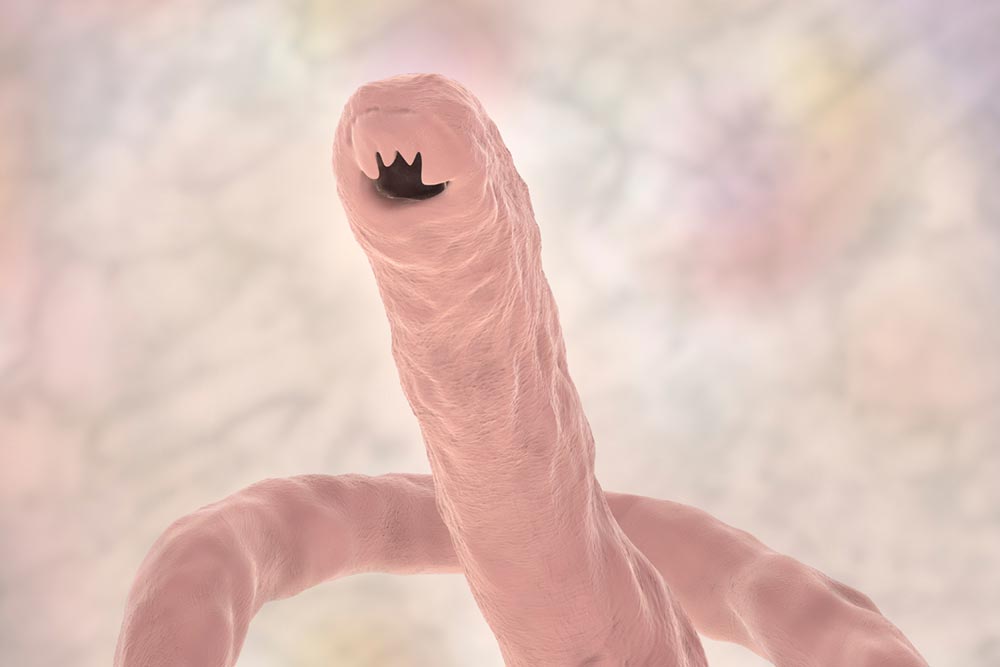
Creating vaccines against parasitic worms, such as hookworms or schistosomes, is arguably even more challenging than developing ones against malaria, because of their size and biological complexity. "The malaria parasite is a single-celled organism, but hookworms are basically animals," says Hotez. "Imagine the complexity of making a vaccine against an animal."
Funding is also an ongoing issue, he says: "Malaria is a killing disease, but a lot of these other parasitic diseases are debilitating, rather than necessarily having a high mortality. Getting people to understand that is a bit more subtle than just saying 200 children die every hour from malaria."
Starvation strategy
Both the hookworm vaccine developed by the Texas Children's Hospital group co-headed by Hotez and Dr Maria Elena Bottazzi, as well as the schistosomiasis vaccines being developed by this group and Tendler's, target the apparatus that these parasites use to feed – either killing them or weakening them to the point where they can no longer produce offspring, meaning they can't infect other people.
"When a woman dies in childbirth in Africa, it is not because she bleeds more, but because she has a low haematocrit [a low number of red blood cells], and that’s because she has combined malaria and hookworm – or sometimes malaria, hookworm and schistosomiasis."
– Prof Peter Hotez, vaccine researcher, Baylor College of Medicine and Texas Children’s Hospital, US.
The Texas Children's Hospital's hookworm vaccine (known as Na-GST-1) targets an enzyme called glutathione-s-transferase-1, which these parasites use to bind and detoxify a component of the haemoglobin protein that red blood cells use to carry oxygen around the body, called haem. Unbound haem damages the hookworms' cells, so directing an immune response against this enzyme means that the hookworms are gradually poisoned by the blood that they feed on. Another hookworm vaccine that is also in human trials (called Na-APR-1) targets a related enzyme called aspartic protease-1, which similarly helps hookworms to safely digest haemoglobin.
So far, phase 1 studies in Gabon, Brazil and the US have confirmed that these vaccines are safe and generate immune responses against Necator americanus, one of the two main species of hookworm that infect humans. The results of a further 'challenge trial' that involved immunising people with the Na-GST-1 vaccine, infecting them with hookworm larvae, and then counting how many eggs could be detected in their faeces, are expected to be published soon.
Meanwhile, schistosomiasis vaccines are also progressing through human trials. Those developed by Tendler's and the Texas Children's Hospital's teams, plus a US-based company called PAI Life Sciences, all target fatty acid binding proteins that these parasites use to absorb fat from their host's blood. "These proteins are of key importance to almost all helminths [parasitic worms], because they are not capable of synthesising fats themselves, which are needed for their survival," says Tendler.
"Malaria is a killing disease, but a lot of these other parasitic diseases are debilitating, rather than necessarily having a high mortality. Getting people to understand that is a bit more subtle than just saying 200 children die every hour from malaria."
– Prof Peter Hotez, vaccine researcher, Baylor College of Medicine and Texas Children’s Hospital, US.
Both the Texas Children's Hospital vaccine and one that Tendler's team has been developing are currently in phase 2 trials, while PAI's vaccine candidate has entered phase 1 trials.
"So far, our results suggest that [our vaccine] induces a very strong and long-lasting immune response, including both cellular and humoral [antibody-based] responses," Tendler says.
Black fever
Leishmaniasis presents a slightly different challenge. Like malaria, it is caused by microscopic single-celled parasites called protozoa, but whereas there are five parasite species that cause malaria in humans, leishmaniasis is caused by at least 20 different species of Leishmania parasite. These are transmitted by more than 90 different species of sandfly, which are difficult to control because, unlike mosquitoes, their breeding sites are difficult to identify, and they are also smaller and quieter than mosquitoes.
Credit: LJNovaScotia from Pixabay
Leishmania can also cause several different types of infection: visceral leishmaniasis (kala-azar or "black fever"), which is characterised by bouts of fever, weight loss, enlargement of the spleen and liver, and anaemia; cutaneous leishmaniasis, which causes skin ulcers that can cause lifelong scarring, disability and stigma; and mucocutaneous leishmaniasis, which destroys mucous membranes lining the nose, mouth and throat.
"We feel confident that we can make our vaccine, scale it, and put it through clinical testing, but will a vaccine manufacturer take it up and what is the business model, i.e. who will do the advance purchasing, and will it be prioritised? That’s not only true of our hookworm vaccine, but I think of the other neglected disease vaccines coming online."
– Prof Peter Hotez, vaccine researcher, Baylor College of Medicine and Texas Children’s Hospital, US.
"Leishmaniasis is actually a large collection of different types of clinical disease, all attributed to different species of Leishmania parasites, which could make it more complicated to develop effective vaccines," says Kaye.
Yet with around one million new leishmaniasis infections occurring each year across 98 countries; almost 20,000 reported deaths; and the threat of further infections because of climate change, urbanisation and growing drug resistance, the case for developing such vaccines remains strong.
Progress is being made towards this goal. Kaye and his colleagues have designed a viral vector vaccine that uses a modified and harmless virus – similar to that used in the Oxford/Astra Zeneca vaccine against COVID-19 – to smuggle genes that code for Leishmania proteins into human cells, so that they manufacture these proteins themselves. Doing so triggers a strong cellular immune response by T cells as well the production of antibodies by B cells.
"So far, the vaccine has been through human phase 1 studies in the UK and a phase 2 safety study in Sudanese patients, to check that there are no untoward effects of vaccination, which there aren't," says Kaye. His team has also just completed a phase two efficacy trial in patients with an unusual skin form of leishmaniasis, called post-kala-azar dermal leishmaniasis, to test whether the vaccine could be used therapeutically, instead of drugs. The results are expected to be published soon.
A live attenuated vaccine against leishmaniasis is also expected to enter human trials later this year.
Roadblocks to success
Exciting as such developments are, there may be further roadblocks ahead. One issue is that many of the researchers who are trying to develop parasite vaccines are doing so in isolation. "What is missing is a formalised roadmap for what we are trying to achieve and the funding that allows people to buy into various aspects of that, like there are for other diseases like malaria or HIV," says Kaye. "There has never been an equivalent for vaccine development against many of the other neglected diseases, including leishmanaisis."
"Vaccines are being developed more as a cottage industry, and I think that's probably something that must change over the next few years if we're going to make progress."
These creatures have spent millennia evolving strategies to stow themselves away in our bodies and steal the resources we need to flourish. It is high time we developed a counter-strategy to defeat them.
Another question is whether manufacturers in low- and middle-income countries will be prepared to invest in producing such vaccines – and whether countries will commit to buying them. Hotez says: "We feel confident that we can make our vaccine, scale it, and put it through clinical testing, but will a vaccine manufacturer take it up and what is the business model, i.e. who will do the advance purchasing, and will it be prioritised? That's not only true of our hookworm vaccine, but I think of the other neglected disease vaccines coming online."
Also unclear is the level of protection that vaccines against parasites might provide, and to whom. Though highly effective at protecting infants and young children against malaria, the current malaria vaccines are designed to be used in combination with other interventions, including insecticide-treated bed-nets.
This may be even truer of other parasitic diseases. Whereas most malaria parasites that infect humans don't infect other animals, some other parasitic diseases are zoonotic – meaning they occur in animals as well as humans. "With malaria, if you vaccinate a substantial proportion of the population, you may be able to break down the transmission [of the parasite] even if you don't vaccinate 100%, whereas with zoonotic infections, you may vaccinate a high number of people, but the disease will continue because it is still being spread from an animal population into humans," says Kelly. "It means you're unlikely to get herd immunity."
Credit: National Institute of Allergy and Infectious Diseases on Unsplash
Hotez believes that, like malaria, other parasite vaccines are likely to work best in combination with other management strategies, rather than replacing them. However, "getting public health officials to understand that may be complicated, because we currently don't have much of a roadmap for that," he says. Also important, will be finding the best age for administering anti-parasitic vaccines, and investigating whether they could be given at the same time as other routine vaccines.
Assuming such obstacles can be overcome, the benefits of such vaccines could be great. Tafadzwa was lucky that his infection was detected early – and hopefully he won't make the mistake of swimming in infected water again – but millions of other people in Zimbabwe and other low- and middle-income countries are living with undiagnosed infections, or life-long complications because of parasites like schistosomes, hookworms, and Leishmania.
These creatures have spent millennia evolving strategies to stow themselves away in our bodies and steal the resources we need to flourish. It is high time we developed a counter-strategy to defeat them.