Two vaccines, one target: How the RTS,S and R21 malaria vaccines work
After decades of development and testing, the malaria vaccines now being rolled out across Africa are the first to ever successfully target a parasite, rather than a virus or bacterium. But how do these revolutionary vaccines work?
- 16 February 2024
- 3 min read
- by Linda Geddes

The Plasmodium parasite that causes malaria is a complex enemy. With a multi-stage life cycle, and the ability to change its protein clothing to hide from the human immune system, developing a vaccine against it has been a huge challenge.
Now, after decades of trying, the world has two vaccines to help protect children against Plasmodium falciparum – the world's deadliest malaria parasite.
Both vaccines trigger the production of antibodies that block sporozoites from infecting liver cells. Those that do manage to break through are targeted by T cells, which are also activated by the vaccines.
Malaria is transmitted from human-to-human through the bites of female Anopheles mosquitoes. The infection cycle begins when elongated and highly mobile forms of the parasite, called "sporozoites", are injected from the mosquito's salivary glands into a person's blood when the mosquito takes a meal.
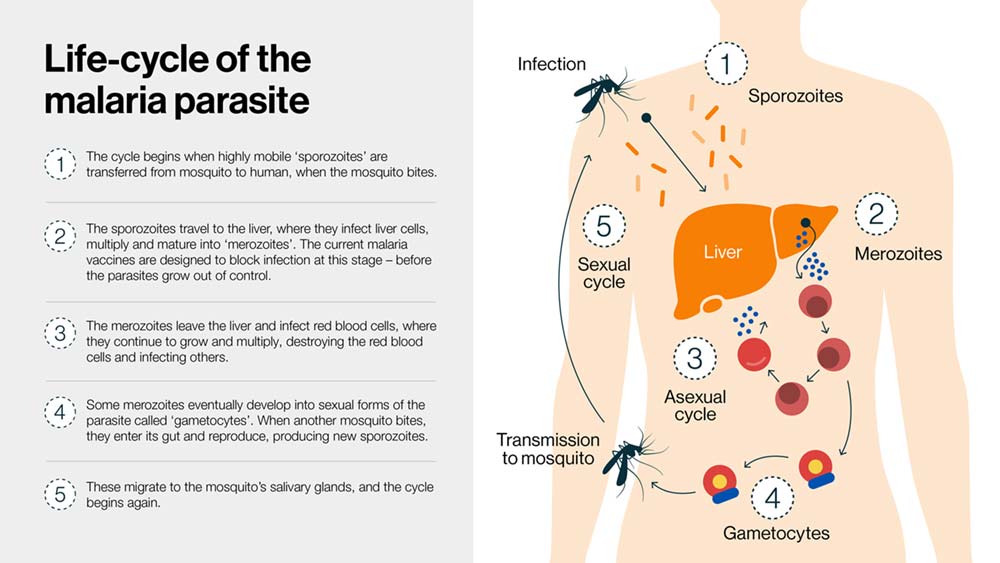
Once inside the body these parasites move from tissue to tissue, repeatedly changing their form (see graphic). But it is the sporozoite stage of their life cycle that both the RTS,S and R21/Matrix M malaria vaccines target; catching the parasite before it has time to grow out of control.
Both vaccines target a protein found on the surface of sporozoites called the circumsporozoite protein (CSP), which helps sporozoites to invade liver cells.
The RTS,S vaccine contains fragments of CSP linked to a protein from the hepatitis B virus that naturally self-assembles into virus-like particles – structures that look like viruses, yet are completely harmless. Linking CSP in this way helps to alert the immune system to it, provoking a stronger vaccine response.
Have you read?
The R21 vaccine also contains fragments of CSP linked to the hepatitis B virus protein, but the manufacturing process differs, resulting in more CSP molecules becoming attached to each virus-like particle.
The vaccines are also delivered alongside different adjuvants – substances that further boost the immune system's response to the CSP protein (the antigen).
Both vaccines trigger the production of antibodies that block sporozoites from infecting liver cells. Those that do manage to break through are targeted by T cells, which are also activated by the vaccines.
Although they have not been tested in a head-to-head trial, "there is no evidence to date showing one vaccine performs better than the other," the World Health Organization (WHO) said.
WHO has granted prequalification status to both vaccines – the final vetting process to ensure that the vaccines used in immunisation programmes are safe and effective – and has said that the choice of product "should be based on programmatic characteristics, vaccine supply, and vaccine affordability" in individual countries.
The hope is that having two vaccines produced at different manufacturing sites by different companies will ensure that there is sufficient vaccine to help protect all children living in areas affected by P. falciparum malaria, saving hundreds of thousands of young lives.