The silent pandemic: how drug-resistant superbugs risk becoming the world’s number one killer
Antimicrobial resistance has been rising for years, but a spike in antibiotic use during the COVID-19 pandemic has accelerated the process.
- 21 November 2022
- 10 min read
- by Priya Joi , Jessica Gergen
When Scottish bacteriologist Alexander Fleming was awarded a Nobel prize in 1945 for his discovery of penicillin, he could already see a future unfold in which life-saving antibiotics would be rendered useless. "It's not difficult to make microbes resistant to penicillin in the laboratory," he warned.
Fleming's nightmare vision has now come true on a terrifying scale. Antimicrobial resistance (AMR) is growing rapidly and is predicted to kill ten million people every year by 2050.
That might mean that "antimicrobial resistance could kill us before the climate crisis does," as Sally Davies, the UK's special envoy on AMR recently warned.
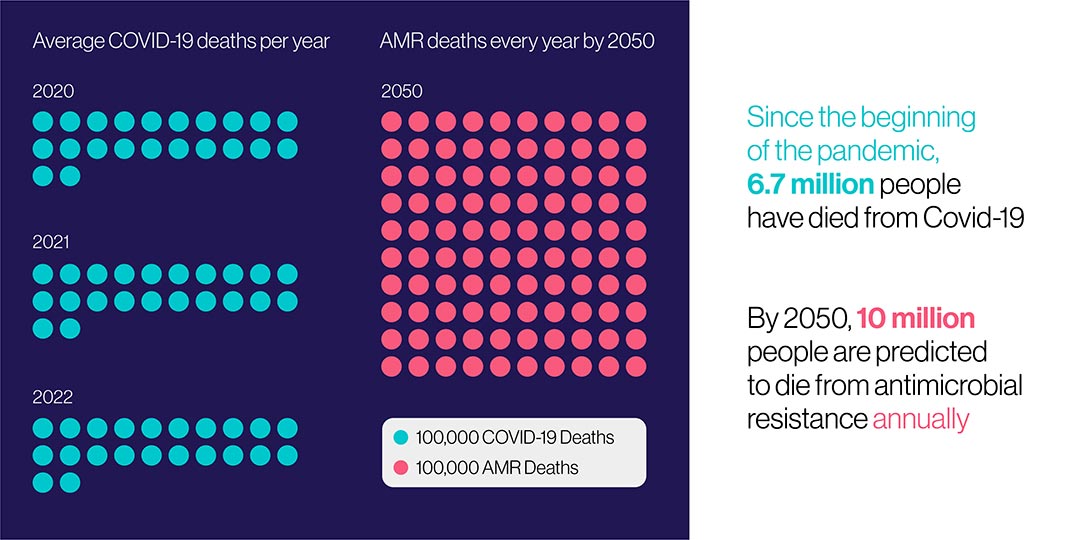
Source: https://amr-review.org
What drives AMR?
Bacteria, viruses, fungi and parasites naturally develop resistance to antimicrobials through evolution. When we use antimicrobials against an infection, the strains of the pathogen that are able to resist them are the ones that are more likely to live and reproduce. This is the survival of the fittest and is a natural process.
But human beings are actually accelerating resistance by using antimicrobials in excessive volumes – in people who don't need them, in the feed of food animals on the land or in the sea, or in pharmaceutical run-off. This puts constant pressure on organisms to evolve to resist those overabundant antimicrobials. Resistant strains are encouraged to thrive.
In places where there is poor water, hygiene and sanitation, poor infection prevention and control, resistant strains can spread further. Where there is a lack of diagnostics, antimicrobials are used when they are not needed. Where people have a lack of access to quality medicine, they might buy substandard antibiotics that don't kill the pathogen, but instead give it the opportunity to develop resistance.
Biggest spike in AMR in low-income countries
Data gathered over the last two decades show growing resistance due to the overuse of antimicrobials, and the use of substandard antimicrobials. This shouldn't be news: scientists have been sounding the alarm bells for a long time now. One of the biggest challenges in tackling AMR is the lack of data gathered on antimicrobial consumption and resistance rates. One data set that researchers can use as a proxy to estimate antibiotic use is data on antibiotics given to treat children for respiratory infections (chart 2).
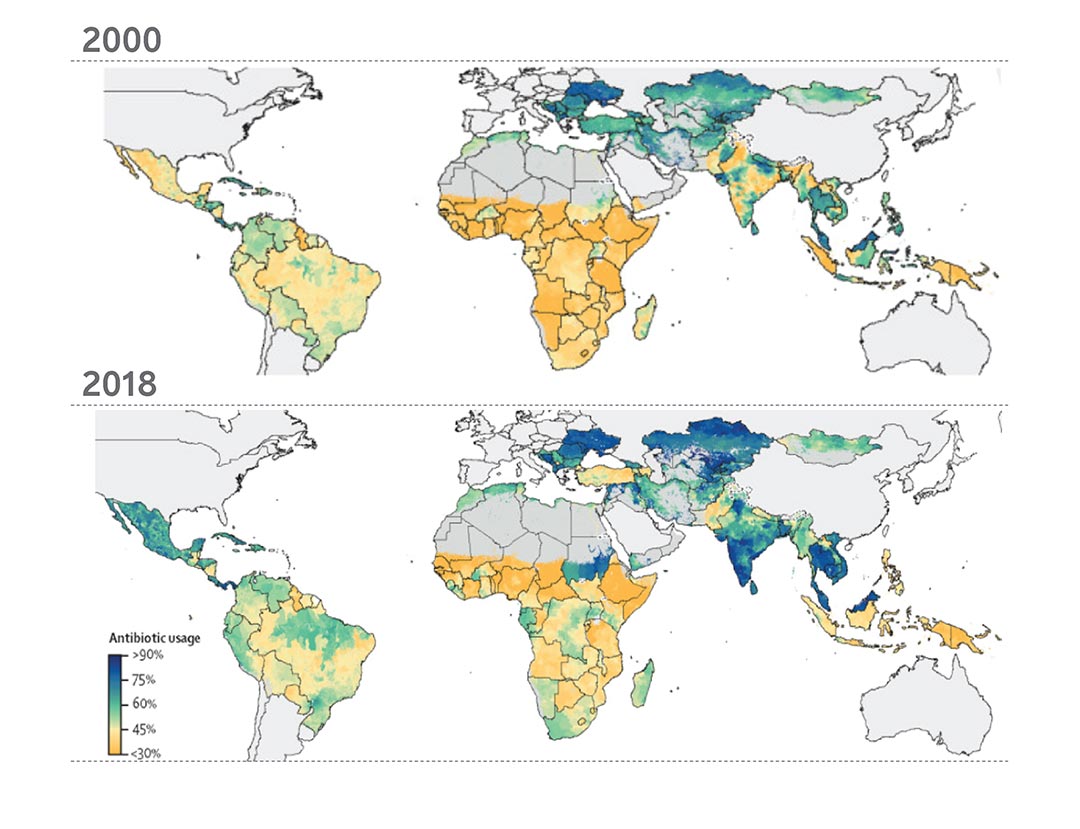
Source: Adapted from a Lancet paper of percentages of children (aged <5 years) with lower respiratory tract infections with caregiver-reported antibiotic usage.
A first-of-its-kind review in the Lancet in 2021 pooled data from a variety of sources to provide a picture of antibiotic use worldwide. In high-income countries, antibiotic consumption mostly stayed the same between 2000 and 2018. Worryingly much of the increase in consumption has been in low-and-middle-income countries (chart 3), where there is lower surveillance of circulating strains and ability to treat resistant infections than in higher income countries. Sub-Saharan Africa saw the biggest increase in consumption, followed by North Africa and the Middle East (chart 3). At the same time, sub-Saharan Africa is seeing the biggest proportion of deaths per population due to AMR (chart 4).
Part of what's bloating antimicrobial consumption in these countries – which in turn is fuelling the AMR crisis – is the inappropriate use of drugs in cases where the disease in question has actually been misdiagnosed. Overdiagnosis of diseases such as malaria, for instance, shows that antimalarials are being prescribed incorrectly in some regions while others have no access at all.
In 2020, researchers studied antibiotic prescription practices in more than 65,000 children under five years in Haiti, Kenya, Malawi, Namibia, Nepal, Senegal, Tanzania, and Uganda. Most of the antibiotics were prescribed to the 80% of kids in the sample diagnosed with respiratory illnesses – and in the lion's share of those cases, antibiotics were unnecessary.
In many low-and-middle-income countries, people are able to buy antibiotics over the counter without a prescription, or are sold counterfeit or substandard antibiotics in poorly regulated markets, further fuelling resistance.
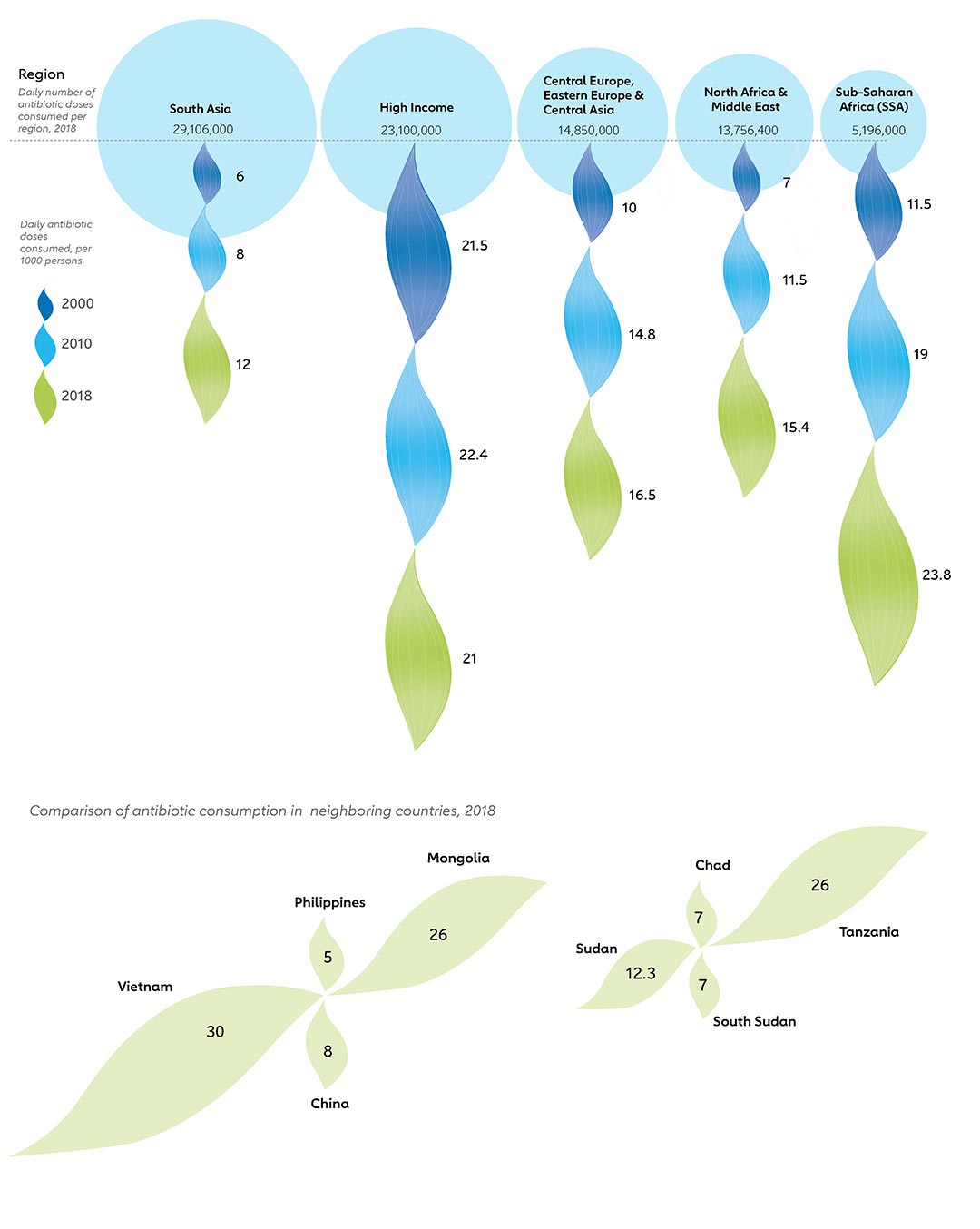
Source: Global antibiotic consumption and usage in humans, 2000–18: a spatial modelling study
More people are now dying from resistant infections than of HIV or malaria (chart 4), according to a 2022 Lancet review of the global burden of AMR. In 2019, 4.95 million people died from illnesses in which antibiotic resistance played a part – in other words, from diseases that were made more difficult to treat as a consequence of AMR, drawing out illness until the point where the patient died. And significantly, 1.27 million people died as a direct result of AMR – because their infection was untreatable – which means that resistant infections killed more people than HIV/AIDS (864,000 deaths) or malaria (643,000 deaths).
The three most common areas of the body for bacterial AMR infections were the chest, bloodstream and abdomen. Infections on these sites accounted for 78.8% of directly attributable AMR deaths.
Have you read?
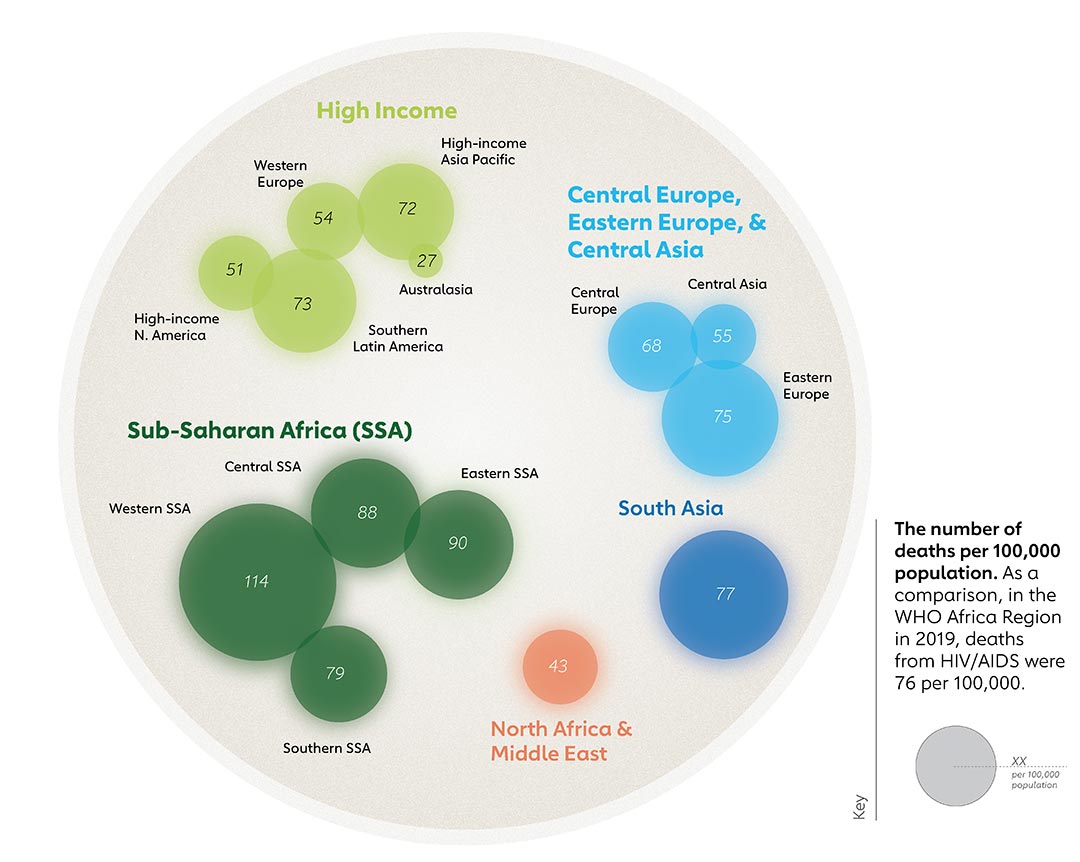
Source
Antimicrobial use shot up in the pandemic
Before the pandemic, AMR was on the rise. Then the pandemic hit, and in the global panic to mitigate the new killer infection, antibiotics were deployed with fresh abandon.
Studies of the use of antibiotics during the pandemic found that 60-70% of hospitalised COVID-19 patient received antibiotics (chart 5), but very few COVID-19 patients (<10% of COVID-19 patients hospitalized) were found to have primary or secondary bacterial infections, meaning most of the antibiotics used were unnecessary.
Factors that led to this pattern of over-use of antibiotics included self-medication and the unavailability of rapid diagnostic tests. What happened next was predictable: increased multidrug-resistance and the worsening of resistance of strains already known to be difficult to treat.
A 2022 systematic review found that more than 90% of studies on rates of AMR published since the start of the COVID-19 pandemic show increased drug resistance, particularly in various species of common gram-negative bacteria (A baumannii, E coli, and K pneumoniae).
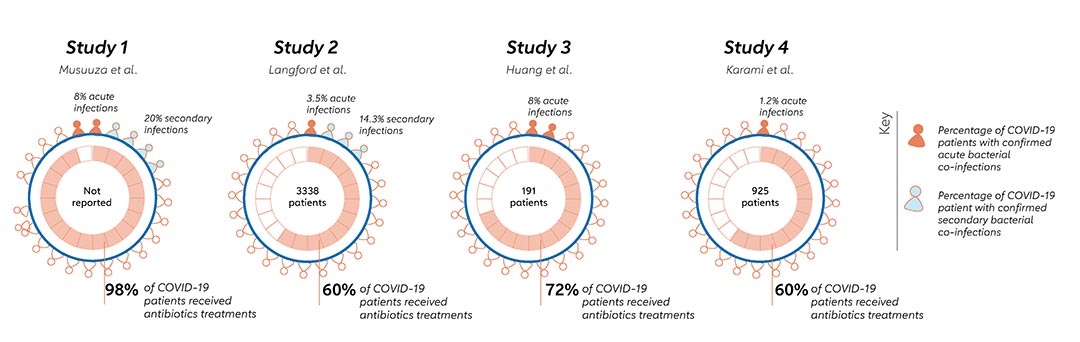
Sources: Jorge et al, study 1, study 2, study 3, study 4
The level of resistance to some of the most common antimicrobials – drugs that are accessible in low-and-middle-income countries, whereas those that can treat resistant infections are not – is approaching 80-90% (chart 6). For example, colistin is an important antibiotic and is the last resort for physicians to treat bacterial infections involving E coli. Resistance to it has been rising during COVID-19, with 20% of K pneumoniae infections now no longer responsive to colistin.
Resistance to fluoroquinolones, carbapenems, cephalosporins, and penicillins – drugs which are often considered to be the first line for empirical therapy of severe infections – accounted for more than 70% of deaths attributable to AMR across pathogens.
Beyond antibiotics, resistance to other antimicrobials such as antimalarials is skyrocketing. The malaria parasite has started to show resistance to artemisinin, one of the main compounds in artemisinin-based combination therapy (ACT) that is the main malaria treatment. However, now there are signs that across Africa, the parasite is developing resistance to the other drugs in the combination, prompting WHO to launch a new strategy to combat this looming crisis.
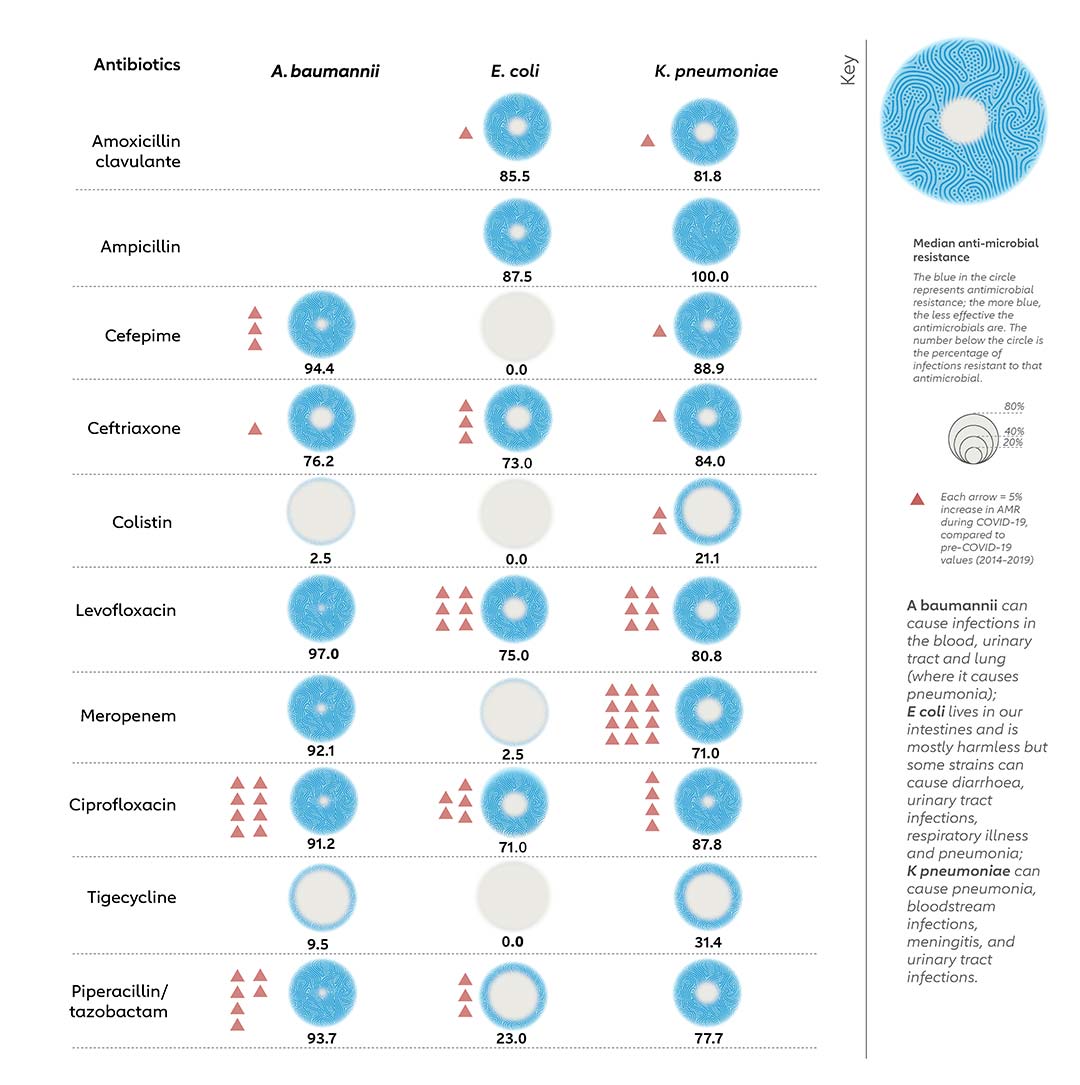
Source: Antibiotic Resistance during COVID-19: A Systematic Review
Value of vaccination
The best way to avoid overusing antimicrobials is to reduce the need for treatment – that is, by preventing infections through immunisation.
Six of the leading pathogens contributing to the burden of AMR in 2019 (E coli, S aureus, K pneumoniae, S pneumoniae, A baumannii, and P aeruginosa) have been identified as priority pathogens in the fight against AMR by WHO.
There is evidence that deploying vaccines can drive down resistance; after the pneumococcal conjugate vaccine PCV-7 was rolled out in the USA in 2000, there was an 84% drop in disease caused by drug-resistant forms of S pneumoniae that were specifically targeted by this vaccine in children under two.So far, however, S pneumoniae is the only priority AMR pathogen that has a vaccine, although vaccine development is underway for the others.
Vaccinating against other pathogens that are developing resistance would contribute greatly to reducing AMR. For example, better deployment of the typhoid conjugate vaccine (TCV) would prevent the spread of strains of typhoid bacterium that are extensively drug-resistant (XDR) and that countries like Pakistan are struggling to treat. Even with vaccines and antibiotics, around 21 million people catch Salmonella typhi each year, and 161,000 die.
Somewhat counterintuitively, even vaccines against viruses can help drive down antibiotic resistance. Given that antibiotics are often wrongly given for the treatment of viral febrile illnesses such as influenza, preventing these infections by vaccination – as well as by other basic infection prevention methods such as wearing a mask or avoiding contact with other people – could reduce pointless over-consumption. In a nutshell: fewer symptomatic people, fewer needlessly consumed antibiotics.
Staving off viral infection can also obviate the more legitimate need to treat bacterial infections that can opportunistically follow viral respiratory infections, say scientists.
Living in a post-antimicrobial world
Although drug discovery is underway for new antimicrobials, this is not an easy task. The consequences of ever-rising AMR are dire. We risk dramatically backsliding in our ability to treat routine infections (chart 7).
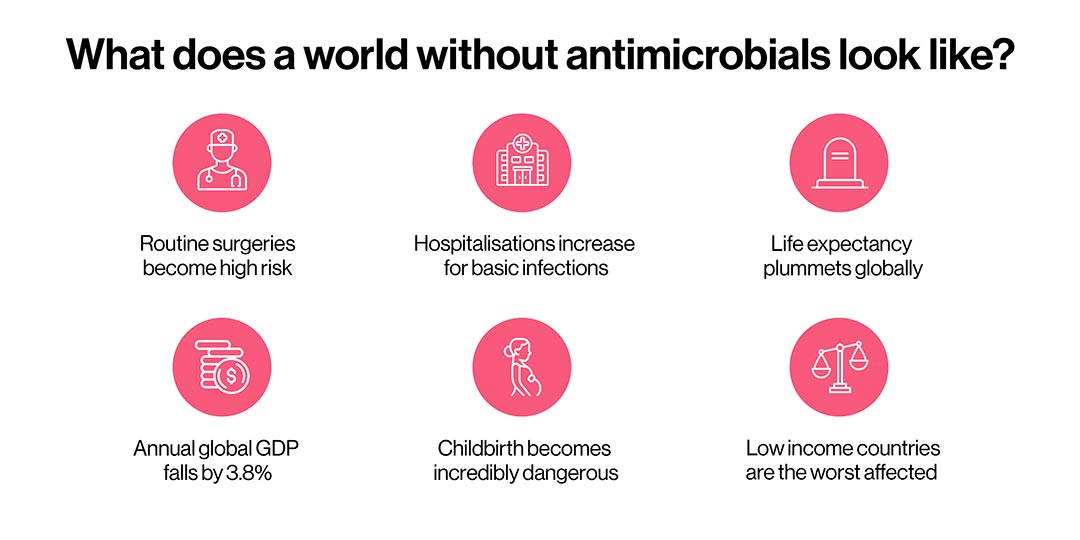
Sources: 1 & 2
In low-income countries, many surgeries could become so risky as to be impossible to perform, because of a lack of sterile facilities and equipment. Worldwide, people would start dying much younger again as we would be unable to treat many infections. It's worth recalling that during the world wars, more young, healthy people died of infections from wounds than of gunshots.
Without the ability to treat infections during or after birth, childbirth, even in high-income countries where it is now safe in most cases, would become incredibly dangerous again. In low-income countries, where 94% of all maternal deaths currently occur, the picture would deteriorate further.
With basic infections becoming untreatable with antimicrobials at home, more people would need to be hospitalised for care. This would increase the burden on hospitals and in countries with high healthcare costs, many would be unable to seek care at all.
The steep financial costs of treating resistant infections are likely to be high. Even in wealthy countries, treatments such as cancer chemotherapy would become prohibitively expensive. As chemotherapy weakens the immune system, people who are undergoing it are highly susceptible to infection; in a highly drug-resistant world, this would mean treatment would become far more complicated as it would require totally sterile conditions, and therefore be extremely costly.
The picture is worse in low-income countries, where people are already at risk of financial ruin because of excessive out-of-pocket payments. AMR could tip an estimated 26 million people into extreme poverty by 2050.
The time for action is now
While tackling AMR is complicated, there is still a shocking lack of political action around it. Many governments are still not taking essential first steps in monitoring or regulating antimicrobial consumption, which makes it hard to expect sufficient action from individual sectors such as health, agriculture and environment – all of which will have a role to play.
The World Health Organization's Global Action Plan on AMR emphasises that vaccines are an important preventative measure in the fight against AMR. Yet when researchers analysed 77 out of 90 national AMR action plans developed by WHO Member States, they found that while 90% mentioned vaccination as a tool against AMR, only half had specific indicators to measure how well vaccine use was being promoted.
Every year, countries assess their AMR capacity, through a process coordinated by WHO, WOAH (World Organisation for Animal Health) and the Food and Agriculture Organization of the United Nations (FAO). According to the 2022 ‘Tracking AMR Country Self-Assessment Survey’ or Traccss, out of 166 countries that responded, 17 had no AMR plan at all, 102 either had one or were developing one, and 30 had a plan that was being implemented but not monitored.
Around 50 of the countries that responded said they have no national system to monitor the use of antimicrobials, and just 36 said they have regularly reported data on the prescription and appropriate use of medicines. Less than half of countries (92) track the use of antibiotics in land or aquatic animals, and this is important to measure as antimicrobial resistant strains in animals or the environment can spread to people.
AMR is as complex and all-encompassing as climate change, and progress against it will inevitably be an uphill battle. Pushing change in human health alone can be challenging, but when policy and process changes need to happen in tandem across human, animal and environmental health, they require extraordinary political and scientific momentum.
For Davies, one of the reasons why AMR still hasn't captured global attention despite the certainty that it will affect every last one of us is that it remains too abstract. "We haven't managed yet to sell it well enough," she told the New Statesman. People dying of AMR aren't visible in the same way that that people who have HIV/AIDS or malaria are visible. It's a silent killer rather than a disease with a recognisable set of symptoms; it kills by making treatments fail. "It's AMR? Huh, so who's dying?" Davies said.
"Immediate and transformational increases in attention and investment are needed," says David Weiss, who studies antibiotic resistance at Emory University in Atlanta, Georgia. The 2022 Lancet review which showed that AMR had played a role in 4.95 million deaths in 2019 was a 'wake-up call', he says: "We cannot wait a minute longer."