What it takes to launch a world-first vaccination trial during an outbreak
The race to ensure an effective vaccine for Sudan virus is on.
- 18 August 2025
- 3 min read
- by Swati Gupta

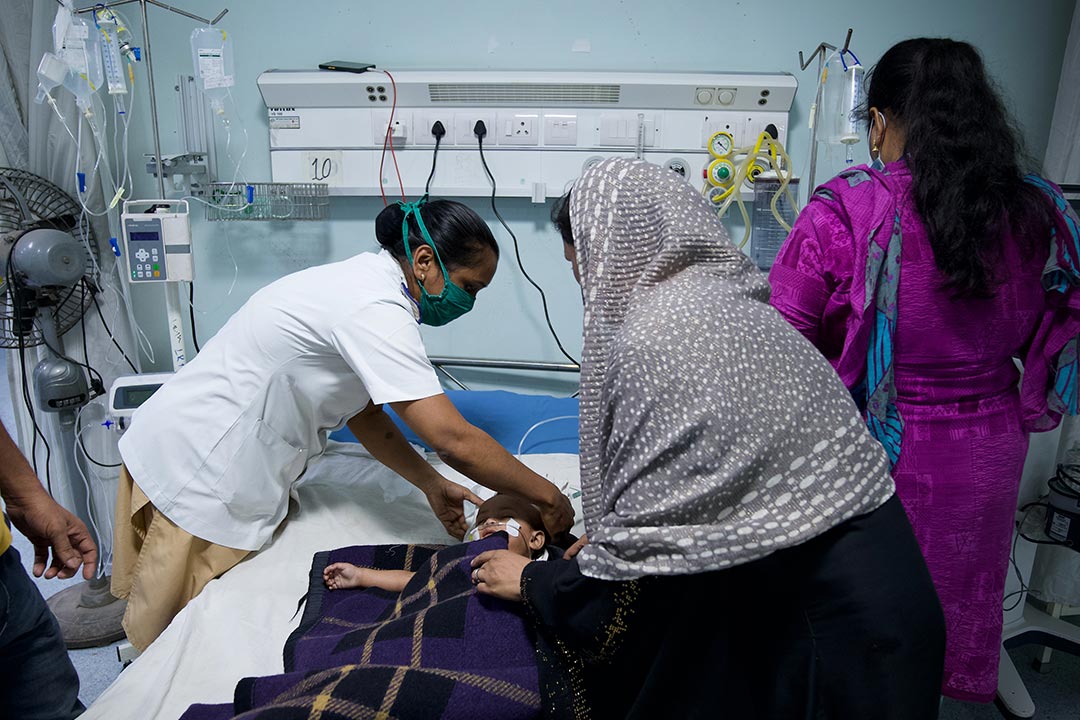
When we received the call on 30 January from the World Health Organization (WHO) about a potential Sudan virus (SUDV) outbreak in Uganda, there was a collective understanding that we had to act fast. Laboratory tests of a Ugandan nurse displaying telltale haemorrhagic fever symptoms confirmed the culprit was SUDV.
This orthoebolavirus causes Sudan virus disease (SVD), a severe, often fatal illness in humans with a case fatality rate of 41–100%.1 No vaccine or specific therapy is available. Instead, local public health teams must isolate suspected cases and their contacts and provide responsive care – often at great personal risk.
Immediately, IAVI, WHO, Ugandan health authorities, the Coalition for Epidemic Preparedness Innovations (CEPI) and other global partners mobilised to deploy IAVI’s investigational SUDV vaccine candidates from an existing stockpile.
Two thousand vials had been prepositioned in Uganda during a previous outbreak in 2022 that ended before a trial could begin. At that time, the WHO Vaccine Prioritization Working Group ranked IAVI’s vaccine candidate highest for use in the trial out of the three vaccines considered. So this time, we were ready with pre-approved protocols, contracts and ethics clearance in place.
Just four days later, on 3 February, the IAVI team and our partners watched with a mix of pride and relief as colleagues at the Makerere University Lung Institute in Kampala administered the first vaccinations as part of the WHO-led “Tokemeza SVD” ring vaccination trial – a world first for an SUDV vaccine. Tokomeza means “to eradicate” in Swahili.
Ring vaccination, a targeted strategy proven effective in past Ebola outbreaks, was used to vaccinate more than 130 volunteers in 17 contact rings as part of a global collaborative response to the outbreak. On April 26, the outbreak was declared over, with no SVD cases reported outside Uganda. The case fatality ratio stood at 29% – lower than in previous outbreaks, but no less devastating for the 14 people infected and their communities.
An effective, accessible vaccine could transform the outlook for communities facing the risk of future SUDV outbreaks. Several candidates are in development, and thanks to its strong track record, IAVI’s SUDV vaccine candidate offers a particularly promising path forward. This single-dose formulation has shown an acceptable safety profile and strong immunogenicity in IAVI-sponsored studies funded by the U.S. Biomedical Advanced Research and Development Authority.2
Its backbone – recombinant vesicular stomatitis virus, or rVSV – is the same platform used in ERVEBO®, Merck’s single-dose vaccine against Ebolavirus, which is licensed in more than a dozen countries, offers high efficacy and durable protection, and has been used extensively in adults and children during Ebola outbreaks.3
As a public–private product development partnership (PDP), it’s IAVI’s mission to ensure that our SUDV vaccine candidate has every chance to make it across the finish line. PDPs like ours act as conveners, and as such, we can more readily absorb the risks of developing preventive vaccines for outbreak pathogens that re-emerge in unpredictable patterns, often in the world’s poorest countries.
Have you read?
While we await results from Tokomeza, emergency support from the Dutch government will enable IAVI and our partners to generate critical safety and immunogenicity data needed to advance our SUDV vaccine toward licensure. Because when the next outbreak comes – and we know from experience that it will – even the most coordinated and rapid response won’t be enough.
There is no substitute for a licensed preventive vaccine that can be administered broadly to protect all those at risk in advance and as outbreaks emerge.
* In 2022, Merck, known as MSD outside the United States and Canada, produced and donated to IAVI vials of rVSV∆G-SEBOV-GP candidate vaccine from existing investigational drug substance to supplement IAVI’s ongoing SUDV vaccine development programme. IAVI now acts as developer and regulatory sponsor and is responsible for all aspects of future development of rVSV∆G-SEBOV-GP.
1 https://www.afro.who.int/health-topics/ebola-disease/sudan-virus-disease