Vaccine patches could transform immunisation coverage in lower-income countries
Micro Array Patches deliver vaccines without syringes, making them easier to distribute and administer. VaccinesWork asked the Developing Country Vaccine Manufacturers’ Network (DCVMN), a group representing manufacturers across the Global South, how they could make a difference in lower-income countries worldwide.
- 6 July 2023
- 4 min read
- by Rajinder Suri , Aila Marini
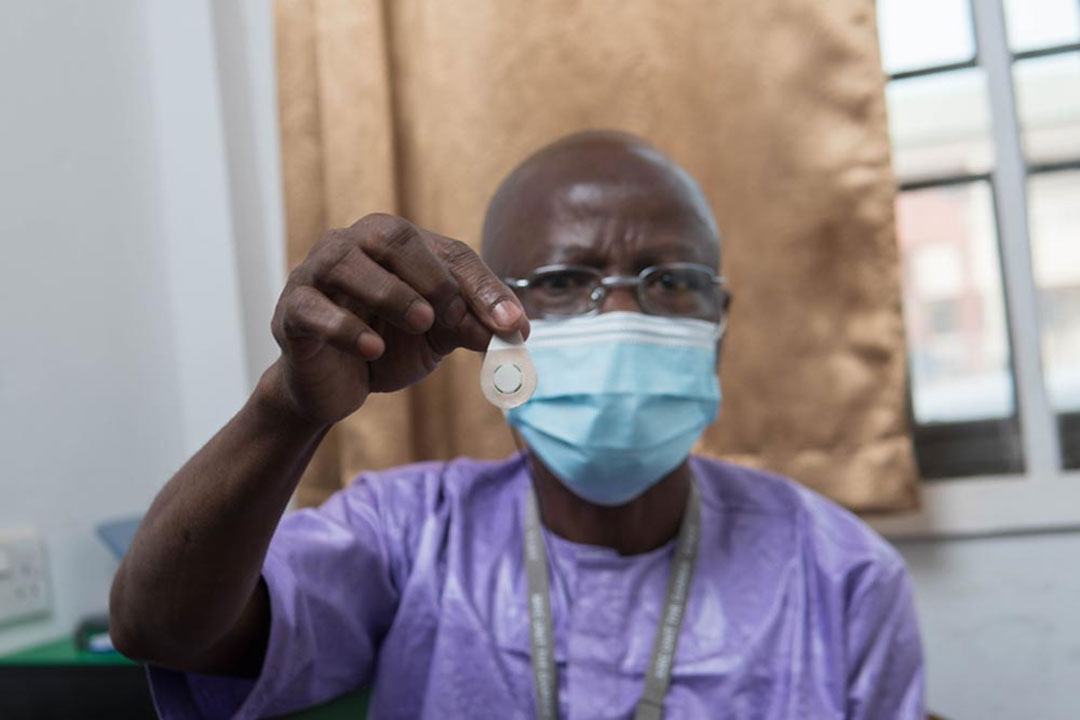
As we work towards reaching zero-dose children and increasing global vaccine coverage, new technologies are being explored to transform the way that vaccines are delivered in low- and lower middle-income countries (LICs and LMICs) to expand their vaccine programmes.
The simplicity, versatility, safety and thermostability advantages make MAPs a promising alternative to intradermal vaccines, allowing for the expansion and improvement of immunisation programmes.
Micro Array Patches (MAPs) have the potential to change the way vaccines are delivered in developing country settings. They are developed to overcome many obstacles and bottlenecks faced by intradermal vaccine delivery, thus maximising the reach of vaccines to the most remote locations to turn vaccines into vaccinations!
The benefits of MAPs in lower-income countries are including but not limited to:
- Being needle-free and pre-dosed, they simplify the administration of the vaccines, as the immunisation can be carried out by minimally trained volunteers, thus addressing the problem of not having trained healthcare workers in remote locations.
- MAPs are safer as they overcome the risks related to operational errors and reconstitution using wrong diluents and/or error in dosages, as well as needle-stick injuries.
- Thanks to their shape and light weight, MAPs are easier to distribute and would also have a smaller wastage footprint.
- MAPs should have enhanced thermostability, thus tackling the problem of vaccine storage requirements and removing the need for cold-chains, which pose a great challenge to traditional vaccination methods.
- MAPs can be a highly cost-effective new vaccine delivery system.
- The lower level of pain experienced during administration with MAPs would help reduce vaccine hesitancy and increase vaccine acceptability.
Regardless of the promise they show, no MAPs have yet been approved by regulators. At the time of writing, one measles and rubella vaccine patch has completed Phase 1/2 clinical trials. Two additional phase 1/2 clinical trials are planned. Some COVID-19 and flu vaccines are also entering Phase1/2 trials, and other vaccines such as HPV are undergoing preclinical assessment.
In May 2023, positive Phase 1/2 data from the first-ever clinical trial of micro-array technology in children was shared during the MICRONEEDLES 2023 conference in Seattle, Washington. The study evaluated the safety, immunogenicity, and acceptability of the measles-rubella (MR) vaccine provided by DCVMN member, Serum Institute of India, which is delivered by Micron Biomedical 's micro-array technology.
Have you read?
The trial was conducted in Gambia, with 45 adults, 120 toddlers (15–18 months old) and 120 infants (9–10 months old) who were randomised to receive MR vaccine either by Micron's micro array or by subcutaneous injection. The Day-42 immunogenicity showed high and similar seroprotection rates for measles and rubella in all cohorts for both the micro array (93.2% - 100%) and SC injection (89.8% - 100%) groups, and similar promising results were seen in infants who were MR-vaccine naïve at the start of the trial.
Many studies have explored the acceptability of MAPs in LMICs by using projection-only MAPs (without vaccine) or backing-only MAPs (without vaccine or projections). One of the studies conducted in Ghana that assessed the usability and acceptability of MAPs demonstrated that all health care workers were able to apply the MAPs correctly and that they felt confident they could successfully apply them in campaign settings. However, monitoring the time that the MAP must be worn for complete immunisation was identified as a logistical challenge.
A study carried out in Benin, Nepal, and Vietnam assessed the perception of MAP acceptability among EPI managers and national stakeholders, health care workers, community representatives and caretakers of children aged 9–23 months. The study determined that the MAPs were accepted with enthusiasm and easy to use, however the administration should still be supervised by the health care worker.
Unfortunately, there are some challenges standing in the way of MAPs becoming widely used. The largest roadblock at the moment is the lack of large production facilities for MAPs, which prevents the scale-up it requires to come to a commercial scale. There is a need for investments to fund pilot-scale manufacturing facilities for MAPs, however this investment is seen as a risk, as there is no guarantee of return.
A way to accelerate production development is to improve knowledge sharing between developers: an example of this is PATH's Center of Excellence for Microarray Patch Technology. As with other vaccine areas, COVID-19 brought MAPs technology to the interest of various stakeholders, permitting the acceleration of MAPs research and clinical trials.
Ultimately, the simplicity, versatility, safety and thermostability advantages make MAPs a promising alternative to intradermal vaccines, allowing for the expansion and improvement of immunisation programmes. At DCVMN, we call for inter-institutional collaborations to scale-up pre-clinical and clinical efforts and enable scalable fabrication to develop the use of MAPs into a common immunisation practice.
Rajinder Suri is CEO-DCVMN International
Aila Marini is Assistant Manager-Communications, DCVMN International






