How effective are malaria vaccines?
When it comes to measuring efficacy, where, when and how you give malaria vaccines matters.
- 29 July 2024
- 6 min read
- by Linda Geddes
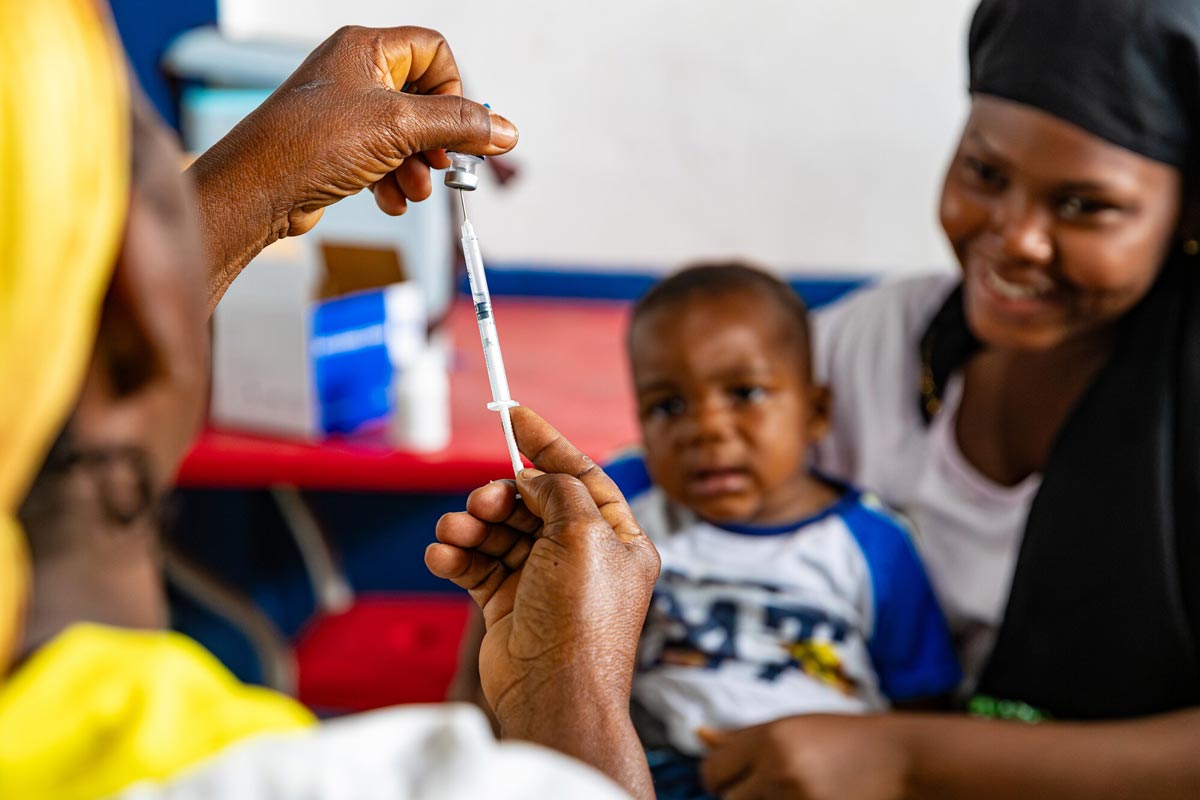
The roll-out of the RTS,S and R21 vaccines against malaria marks a turning point in the millennia-old battle against this deadly infection. Both vaccines work by targeting the same protein on the surface of malaria parasites and are expected to have a substantial impact on reducing malaria cases and deaths in children.
Interpreting their efficacy numbers – a measure of how much a vaccine protects people against disease under controlled trial conditions – can be challenging. This is because these measurements are not directly comparable due to differences in study design and contexts – using different approaches to provide the vaccine, measuring the vaccine’s effect over varying lengths of time or in areas with differing malaria burden.
While both RTS,S and R21 studies have shown that efficacy is improved when using a seasonal approach, running public health campaigns to deliver vaccines seasonally is much more complex to implement in practice compared to an age-based approach.
RTS,S vaccine clinical trials were conducted in many sites and have seven years of follow-up data, which means far more is known about RTS,S efficacy in different settings, and how long the protection it provides lasts for. Efficacy results for the R21 vaccine are based on 12 months of follow-up and fewer study sites.
Here’s a summary of what we know about the efficacy of malaria vaccines so far.
The efficacy numbers
The large-scale phase 3 trial of the RTS,S vaccine evaluated its efficacy from 2009 to 2014 across 11 different study sites in seven countries: Burkina Faso, Gabon, Ghana, Kenya, Malawi, Mozambique and Tanzania. These sites represent a range of malaria transmission intensities across sub-Saharan Africa . A substantial number of malaria cases were prevented in all countries when children were vaccinated in an age-based approach: the children received three vaccine doses starting from around 5 months of age, plus a fourth dose to prolong protection around age 2.
The RTS,S vaccine reduced the number of malaria cases by half (51%) during the year following vaccination, and this included in study sites in low and high-transmission areas.
A later trial of RTS,S using a different vaccination strategy found that, for countries with highly seasonal patterns of malaria transmission, giving the initial three vaccine doses before the start of the peak “rainy” season (seasonal administration) was even more efficacious – at 72% over the first year. Additional “booster” doses administered each year just prior to subsequent malaria seasons prolonged the protection.
The phase 3 clinical trial of the R21 vaccine started in 2019, and was conducted in five study sites in four countries – Burkina Faso, Mali, Kenya and Tanzania. This trial evaluated an age-based vaccination approach in three of the study sites and seasonal vaccination in two study sites. Similar to RTS,S, when given in an age-based approach, R21 reduced clinical malaria by 66% in the 12 months following vaccination.
Age-based vaccination in the R21 trial included study sites in low to moderate transmission areas but did not include sites with year-round high transmission settings.
Similar to the RTS,S seasonal vaccination trial, when the R21 vaccine was provided just before the malaria season in areas of seasonal transmission, malaria episodes were reduced by 75% in the first year following vaccination.
Of particular importance to countries aiming to roll out the vaccine is the fact that, while both RTS,S and R21 studies have shown that efficacy is improved when using a seasonal approach, running public health campaigns to deliver vaccines seasonally is much more complex to implement in practice compared to an age-based approach where vaccines are given to children through the routine system at childhood immunisation clinics.
Transmission intensity and impact
Results from the large-scale RTS,S study showed that lower transmission areas tended to have higher efficacy numbers, but the vaccine achieved higher impact in reducing malaria cases in high transmission areas, where the burden is highest.
This is important because neither malaria vaccine provides complete protection against infection . Both vaccines target malaria parasites during the ‘sporozoite’ stage of their lifecycle – the point where they first enter the human body and begin replicating in liver cells.
“In areas – and time periods – with high transmission, the immune system is under a significant assault due to repeated biting from mosquitos,” says Dr W. Scott Gordon, Head of Gavi’s Malaria Vaccine Programme. “Each bite can transmit as many as 1,000 sporozoites, so even a very small proportion of those getting through can result in the immunised child still coming down with malaria, and lower measured efficacy from the vaccine.”
“This is one reason why WHO prioritises the use of malaria vaccines in moderate to high transmission areas, where public health impact is expected to be highest,” says Dr Lindsey Wu, Technical Officer at the WHO Global Malaria Programme.
“While clinical trials are useful for measuring vaccine efficacy, it was through the pilot implementation of RTS,S, where the vaccine was rolled out into a larger population through routine health systems, that we were really able to measure effectiveness in a real-world setting and say ‘this had high public health impact’.”
- Dr Lindsey Wu, Technical Officer at the WHO Global Malaria Programme
Duration of protection
Malaria is most dangerous during the first year of life.
The longer-term follow-up of RTS,S efficacy shows waning of protection over time, which is also shown for other vaccines like COVID-19 vaccines. Children continue to benefit from the RTS,S vaccine over seven years of follow-up.
“Even with moderate efficacy, the currently recommended malaria vaccines provide protection to children during the most vulnerable time of their life,” says Wu.
“We don’t know what the duration of protection with R21 is, because it hasn’t been studied for long enough yet,” said Prof Brian Greenwood, at the London School of Hygiene and Tropical Medicine, UK, who has been involved in phase 3 trials of both vaccines. R21 clinical trials are ongoing.
Public health results: drop in severe malaria and death
Not all children who fall sick from malaria develop severe illness, but for those that do, the consequences can be dire, with death sometimes occurring within 24–48 hours from the start of symptoms, which usually start within 10–15 days of infection.
Pilot introductions of the RTS,S vaccine, through the Malaria Vaccine Implementation Programme, vaccinated more than 2 million children in Ghana, Kenya and Malawi from 2019 to 2023. The evaluation of the large-scale roll-out measured a substantial reduction in severe malaria, and 13% drop in deaths from all causes among children age-eligible for vaccination.
“While clinical trials are useful for measuring vaccine efficacy, it was through the pilot implementation of RTS,S, where the vaccine was rolled out into a larger population through routine health systems, that we were really able to measure effectiveness in a real-world setting and say ‘this had high public health impact’,” says Wu.
For R21, the overall levels of severe malaria and death in the phase 3 trial were too low to measure the effect that the malaria vaccine had on these outcomes.
Have you read?
Impact
So, where does this leave us?
The bottom line is that there are now two safe and effective malaria vaccines available in Africa that will ensure millions more children will have access to this malaria prevention.
With more than 600,000 deaths from malaria each year – the vast majority among African children under the age of five – wide roll-out of both vaccines is expected to save tens of thousands of lives each year.








