Keeping vaccines cool with cold chain
With millions of COVID-19 vaccines now being delivered around the world, they are utilising a huge hidden infrastructure, built up over decades and spreading across the globe, with one ultimate goal: keeping the vaccines cool. How did these vaccine “cold chains” evolve, and why are they so important?
- 31 May 2021
- 5 min read
- by Tetsekela Anyiam-Osigwe

It was the morning of 24 February 2021 and 600,000 doses of the Oxford-AstraZeneca COVID-19 vaccine from the Serum Institute of India had just arrived at the Kotoka International Airport in Accra, Ghana as refrigerated cargo. It was an historic moment - COVAX had just completed its very first international delivery. Yet, this was only the beginning of what would be a chain of coordinated events in temperature-controlled environments to safely deliver these vaccines to those who needed them the most.

Credit: UNICEF/UN0421535/Kofi Acquah UNICEF/UN0421535/Kofi Acquah
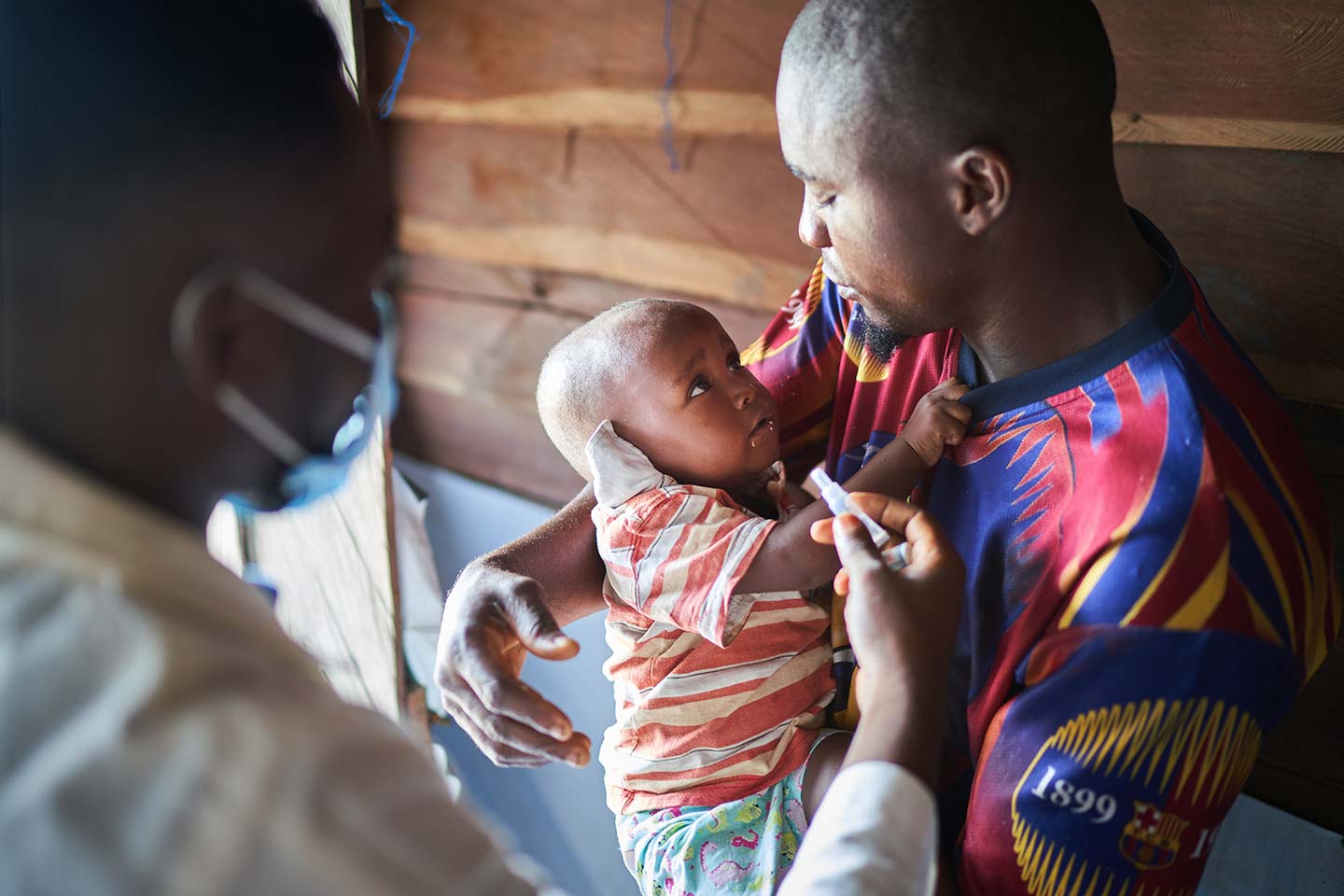
At Kotoka, the vaccines were loaded onto refrigerated trucks and taken to the government's vaccine central storage facility, where they were quickly stored in huge walk-in refrigerators in preparation for the impending roll-out. In a few days, health workers carried the COVAX doses in cold boxes and vaccine carriers, travelling across Accra to vaccinate priority populations against COVID-19. This was the cold chain in action.
Why do vaccines have to be kept cool?
The delivery of COVAX doses is not the only Ghana-first event in the history of global health. Back in 1974, the World Health Organization (WHO) established the Expanded Programme on Immunization (EPI). In order to assess the feasibility of a single, global immunisation schedule incorporating vaccines against polio, measles, tuberculosis, tetanus, diphtheria and pertussis, the EPI was initially piloted in Ghana.
One major challenge quickly became apparent: as vaccines were temperature-sensitive biological products, they needed to be continuously stored in a limited temperature range from the time they were manufactured until the moment of vaccination. Any exposure to temperatures outside this range would irreversibly affect vaccine potency, diminishing the ability of vaccines to protect vaccinated patients.
The solution was a workable “cold chain". A term adopted in 1976, it described a global network of cold rooms, cold boxes, vaccine carriers, refrigerators, and freezers that kept vaccines at the WHO-recommended temperature during the long journey from the manufacturing line to the syringe.
A story of technology and innovation
A manual temperature recording procedure with a mixed compliance record, coupled with unreliable temperature reports, provided the impetus for an innovation drive to create an end-to-end temperature monitoring system for vaccines at every point in the cold chain.
Have you read?
These innovative solutions really started moving at pace in the 1970s and 1980s. Working with a wooden cold box developed by a laboratory in Sweden in 1974, Electrolux Luxembourg developed a vaccine storage container that could be transported easily. The Luxembourg cold box was successfully tested in Ghana, before becoming a widely used model for manufacturers. Three years after the invention of the cold box, a manufacturer of portable containers based in the United States collaborated with the Pan-American Health Organization (PAHO) to create a vaccine carrier, which has now become an integral part of outreach immunisation around the world.

Credit: Gavi/2006/Indras Getachew
By the late 1970s, PATH also began working with the WHO and different technology partners in search of a way to better assess whether vaccines have been heat-damaged. Temperature-sensitive vaccine vial monitor (VVM) stickers, which change colour if they become too warm (meaning the vaccine they're attached has become ineffective), were finally developed in the early 1980s through PATH’s collaboration with the Temptime Corporation. By 1996, these VVMs became available for the oral polio vaccine, and today, the WHO requires all UNICEF-procured vaccines to use VVMs.
Companies in the United States and Switzerland went on to create a cold chain monitor (CCM) in the early 1980s, an innovation that finally gave the global immunisation community a reliable way to monitor vaccine stores at all levels, from manufacturer to delivery sites in different countries.
The chilling effects continue
Even more technology has emerged over time, with cold chain systems increasingly adapting to lower-income country settings: refrigeration and freezer units have become more portable and with digital remote monitoring systems now a common feature, so they can not only be carried on foot to hard-to-reach populations but stock levels can be managed more effectively. Refrigeration equipment also use battery power or solar energy, overcoming the challenge of unstable electricity.
More importantly, these technological innovations have been progressively matched with major investments in human capital through sophisticated training for health workers and technicians to enable them to better manage the vaccine cold chain.
Cold supply for hot demand
Whilst the world was unprepared for the COVID-19 pandemic, it was far more prepared for a large-scale vaccine rollout, thanks to the transformed cold chain systems that have been growing since the early days of the EPI. Previous investments by Gavi, for example, built up a cold chain infrastructure in many lower-income countries, which facilitated the movement of vaccines from production lines to people’s arms, and meant that the distribution of COVID-19 vaccines could proceed, in most cases, without delay.

Credit: Gavi/2020/Isaac Griberg
Keeping our cool
While some countries still do not have the capacity to meet the ultra-cold chain requirements of certain COVID-19 vaccines which need to be stored at deep freeze temperatures of between -75 and -80 °C, this does not mean that ultra-cold chain distribution is impossible. It was carried out successfully for the Ebola vaccine, which also needs to be stored in ultra-cold temperatures, in the Democratic Republic of the Congo (DRC) and neighbouring countries, and because of the scale of the COVID-19 pandemic, countries are using this as an opportunity to strengthen and advance their cold chain capacity.