Seven vital questions about RNA Covid-19 vaccines
The Pfizer-BioNTech and Moderna Covid-19 vaccines are more than 90% effective, as reported in phase III clinical trials – and the Pfizer-BioNTech vaccine is the first Covid-19 vaccine to be licensed.
- 30 December 2020
- 7 min read
- by Wellcome
The Pfizer-BioNTech and Moderna Covid-19 vaccines are more than 90% effective, as reported in phase III clinical trials – and the Pfizer-BioNTech vaccine is the first Covid-19 vaccine to be licensed. This is a historic moment and the result of a global, collaborative research effort.
Both vaccines, unlike the vaccines we already use for other diseases, have been developed using ribonucleic acid (RNA) technology. So, how do they work, are they safe, and when will they be available?
1. How do the vaccines work?
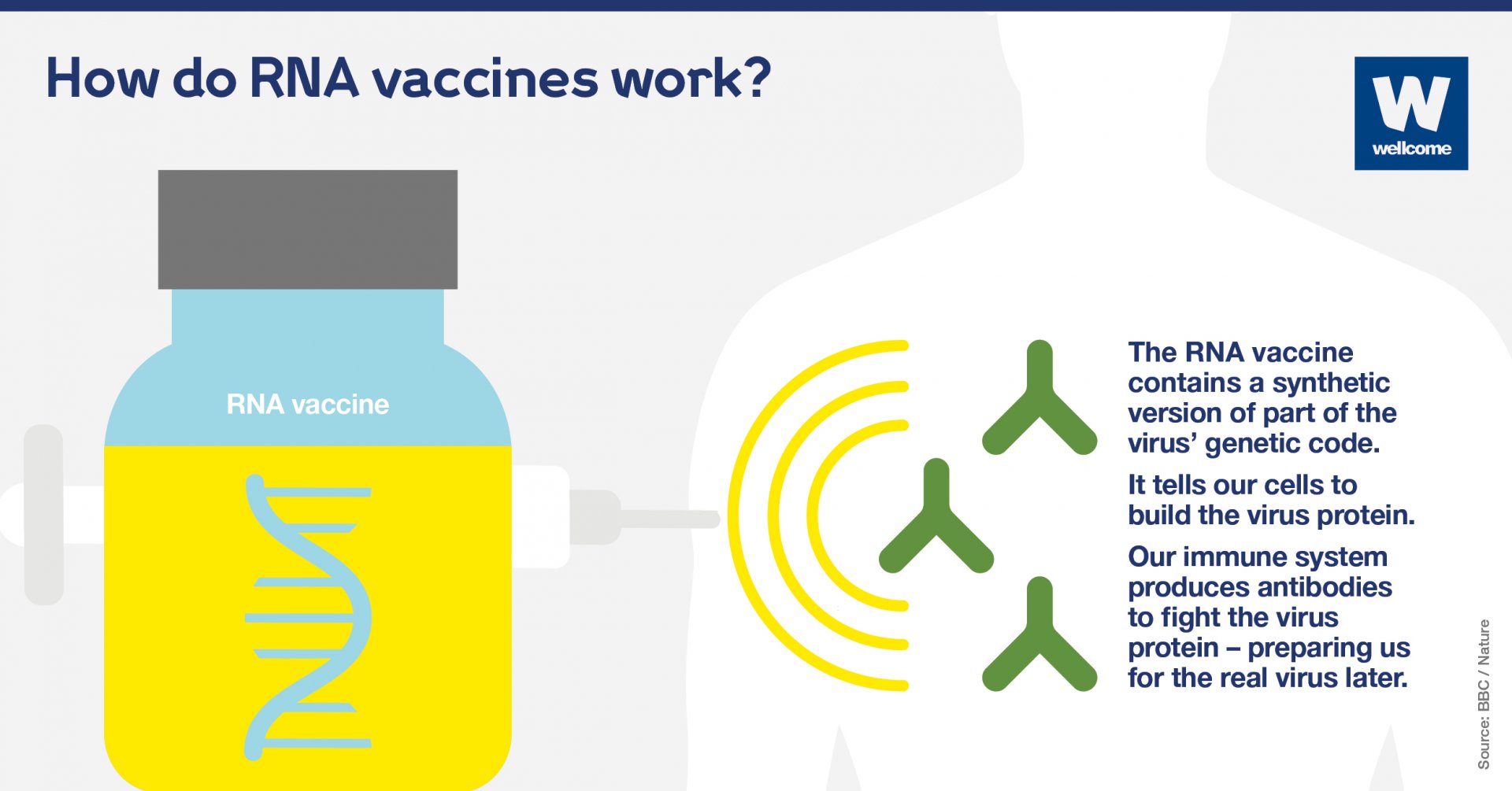
RNA, closely related to DNA, is present in all living cells. The strand of it called messenger RNA is a sequence of genetic code that tells cells what proteins to build so that they can function.
To produce an RNA vaccine, scientists develop a synthetic version of some of the virus’ messenger RNA.
When this is injected into the human body, our cells read it as an instruction to start building the proteins, including, in this case, Covid-19's distinctive 'spike' protein.
Our bodies then mount an immune response by producing antibodies to fight the virus proteins made by our cells. This prepares our immune system to fight the real virus if we encounter it later on.
This is different to the way some other vaccines work, where a small part of the virus itself, or the whole virus (weakened or dead), is injected into the body to trigger an immune response.
2. How is RNA vaccine development different to other vaccine development?
RNA vaccines hold the promise of being faster, cheaper, more adaptable and easier to mass-produce than other vaccines, because:
- They can be generated quickly. RNA vaccines are based on a process of biochemical synthesis that involves fewer components and fewer steps than the more complex traditional methods, like using inactivated live viruses. This means they are quicker to get into clinical trials and quicker to manufacture once the trials are completed – in a matter of weeks and months.
- They should be cheaper to develop. Only a small amount of RNA needs to be delivered into the body’s cells, compared to the much larger micrograms of protein that are required for many other vaccines. This means each individual vaccine dose should be cheaper to purchase, although it is dependant on the price set by pharmaceutical companies and the costs of delivery.
- They could be more adaptable and easier to manufacture at scale. The same RNA vaccine platform could be used to produce vaccines against different diseases – both known and emerging. A manufacturing plant could, in theory, produce multiple vaccines using the platform, whereas other vaccines, such as MMR (measles, mumps, and rubella) and Ervebo (one of the Ebola vaccines), each require their own dedicated manufacturing plant.
3. Have there been any other RNA vaccines?
There are no other licensed RNA vaccines – the Pfizer-BioNTech vaccine is the first.
However, researchers have been using the technology for a while, and people have been given RNA vaccines in clinical trials for other diseases, like cancer.
A major challenge in the past has been figuring out how to deliver the RNA vaccine into the cell so it survives – our bodies naturally want to destroy foreign RNA molecules.
This new use of RNA has only been made possible due to the enormous level of research funding and focus during the pandemic, which has allowed breakthroughs in new technologies. The cutting-edge method could revolutionise vaccine development for future disease outbreaks.
4. Are the vaccines safe?
Before any vaccine can be approved for use it must go through rigorous testing, to make sure it is safe, as well as effective.
During clinical trials, the Pfizer-BioNTech vaccine has been given to around 43,500 people, and the Moderna vaccine to around 30,000 people. Throughout the phase I, II and III trials, safety has been assessed and no major side-effects have been reported so far.
Once the trials are complete and the full data has been analysed, regulators around the world will then review it and decide whether the vaccines can be approved for use in their countries. They look at all the preclinical, clinical and manufacturing process data, including the safety and efficacy data. The Pfizer-BioNTech vaccine has been approved for use in the UK by the Medicines and Healthcare products Regulatory Authority, with more countries due to follow.
Once licensed, the vaccines will be monitored as they are given to prioritised high-risk groups, to understand how they perform in different population groups over time, and to look for very rare side-effects or long-term safety issues.
Nothing in medicine is 100% safe, and very rare side-effects may emerge as millions of people are vaccinated. This is what happens with all vaccines.
5. When will the vaccines be available?
Pfizer-BioNTech have said that they will be able to supply 50 million doses by the end of this year and around 1.3 billion by the end of 2021.
If licensed*, Moderna has said it intends to provide the US government with 20 million doses by the end of this year, and manufacture between 500 million and one billion doses globally throughout 2021. *The Moderna vaccine was granted emergency-use authorisation on 18 December 2020 by the US Food and Drug Administration (FDA).
However, these vaccines are far from being the only hope: there are currently more than 320 Covid-19 vaccine candidates in development. Several of them, including the Oxford/AstraZeneca vaccine, are emerging from phase III trials, so we can expect more announcements like this soon.
6. What are the potential limitations of the vaccines?
The announcements are really positive news. But there are still many outstanding questions, for example how long immunity will last for, how effective the vaccines will be in different populations, and whether people can still transmit the disease to others if they’ve been immunised.
We do know that both Pfizer-BioNTech and Moderna have reported high levels of vaccine efficacy in over 65 year olds – one of the groups most at risk of serious illness – which is very promising.
Although many vaccines need to be refrigerated – usually around 2 to 8C – the Pfizer-BioNTech Covid-19 vaccine needs to be stored at at least -70C, which could pose problems for transporting and storing it, particularly in low- and middle-income countries where refrigeration facilities may be limited.
The Moderna vaccine can be stored at fridge temperature for 30 days (2 to 8C) once delivered to healthcare facilities which is encouraging, but requires -20C for long-term storage and transportation.
It is critical that we continue with efforts to ensure fair access to Covid-19 vaccines, for example through the Covid-19 Vaccine Global Access Facility (COVAX), which will be instrumental to ensuring effective vaccines are prioritised for those most in need globally.
7. Why do we need to continue to invest in different vaccine approaches?
Two Covid-19 vaccines won’t be enough. The world needs a range of vaccines that have different characteristics, suitable for people of all ages and ethnic groups, including people with underlying health conditions, and able to be distributed and used in all settings around the globe. They must also be available in billions of doses. No one or two vaccines will be able to achieve this, and so we must continue to develop multiple vaccines using multiple scientific approaches to be able to control the pandemic.
Over the past decade we’ve seen the health and economic impact of influenza, SARS, Zika, Ebola and now Covid-19. There are certainly more outbreaks to come, but we don’t know when and where they will emerge, which makes preparation difficult. If we can hone new methods of developing vaccines, we’ll be much better prepared for any future outbreaks and able to save more lives with vaccines, faster.
That is why the urgent funding gaps in the global response to Covid-19 must be addressed. Only through appropriate funding can innovations like RNA vaccines be made possible.
Original article
This article was originally published by Wellcome on 13 November 2020 (updated 2 December 2020)