Six things you need to know about how vaccines fight antimicrobial resistance
Vaccines stop infections and can stop antimicrobials being used unnecessarily; a Wellcome report outlines the type of data we need now.
- 1 July 2025
- 6 min read
- by Priya Joi
Vaccination is a critical intervention in the fight against antimicrobial resistance (AMR), yet its potential remains underexploited globally.
Vaccines work by preventing both drug-sensitive and drug-resistant infections, thereby reducing the overall burden of AMR-related diseases.
Current and new vaccines could avert over half a million AMR-associated deaths annually, save US$ 30 billion in hospital costs and reduce antibiotic use by 2.5 billion doses each year.
This dual action is significant: by preventing infections, vaccines reduce the necessity for antimicrobials, which is a primary driver of resistance development.
The World Health Organization estimates that current and new vaccines could avert over half a million AMR-associated deaths annually, save US$ 30 billion in hospital costs and reduce antibiotic use by 2.5 billion doses each year.
Despite these compelling figures and the inclusion of vaccination in 87% of national AMR action plans, implementation often lags due to competing health priorities, limited resources and insufficient integration of immunisation and AMR strategies.
To generate robust evidence on the impact of vaccination in AMR, in 2019 the Wellcome Trust launched a call for proposals that resulted in 13 projects being funded, focusing on different settings, pathogens and experimental approaches.
In a new report, Wellcome summarised new primary evidence generated by 11 of these projects spanning multiple vaccines and settings, which makes clear that the impact of vaccines on AMR is complex. The report offers key recommendations on how improved data should be generated, and what is urgently needed.
The report stresses that vaccines should be viewed not just as disease-prevention tools, but as essential components of AMR control, capable of preserving antibiotic efficacy for future generations.
Their broad-spectrum impact, affecting both resistant and susceptible strains, means that even incremental increases in vaccine coverage can yield substantial public health benefits.
1. Vaccines mitigate AMR through multiple pathways
Directly, they prevent infections caused by resistant pathogens, thereby reducing the incidence of hard-to-treat diseases. Indirectly, by lowering the overall infection burden, vaccines decrease the need for antibiotics, which in turn reduces the selection pressure that drives resistance. However, the magnitude and consistency of these effects are highly variable.
Wellcome research showed that in Guatemala, rotavirus and pneumococcal conjugate vaccine (PCV) vaccination was associated with reduced prevalence of extended-spectrum cephalosporin-resistant enterobacterales in the gut; this seems to have been mediated mainly by a reduction in the associated clinical syndrome rather than reduced antimicrobial use.
An ongoing cluster-randomised study in Malawi found that both a modified PCV13 schedule (2 +1) and the RTS,S/AS01 malaria vaccine were associated with reduced antimicrobial use and less resistant bacterial isolates in young children; however, use of malaria rapid diagnostic tests was associated with increased likelihood of antimicrobial prescription. It’s not clear what drives this, but it could be that after a negative malaria test where a fever remains, antimicrobials seem the best option.
For example, typhoid and pneumococcal vaccines have demonstrated reductions in AMR, but the relationship is complicated by factors such as serotype replacement (where non-vaccine strains fill the ecological niche) and local prescribing practices.
2. Some evidence for vaccine impacts on AMR is stronger than others
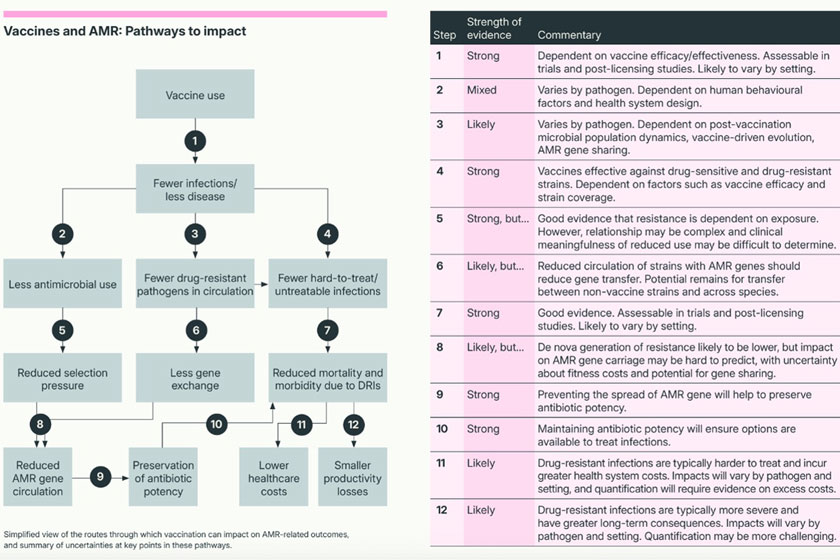
The report highlights that the effects of vaccination on AMR are mediated by a complex “pathway to impact,” which includes microbial population dynamics, human behaviour, health system design, and even economic incentives.
This complexity means there is rarely a straightforward, linear relationship between vaccine use and measurable reductions in AMR (see figure).
As a result, policymakers and researchers must consider local context, including pathogen prevalence, vaccine coverage and health system factors, when assessing the likely impact of vaccination on AMR outcomes.
3. Robust, practical evidence for vaccine impact on AMR is urgently needed
While modelling studies have provided valuable estimates of the potential impact of vaccines on AMR, there is a pressing need for more empirical, real-world data, especially from low- and middle-income countries (LMICs) where the burden of AMR is often highest.
The Wellcome-funded projects reviewed in the report encompass a wide range of settings, pathogens, and methodologies, revealing significant complexity in the relationship between vaccination, antimicrobial use, and AMR. For instance, studies on influenza, typhoid, pneumococcal, malaria, and diarrhoeal diseases show that vaccines can reduce antibiotic prescribing, but the effects are inconsistent and influenced by local health system factors, diagnostic practices, and patient behaviours.
Furthermore, even when reductions in antibiotic use or AMR gene prevalence are observed, the biological and clinical significance of these changes is not always clear. The report underscores the importance of embedding AMR endpoints in vaccine trials and observational studies, and of strengthening surveillance systems to generate robust, context-specific evidence that can inform policy and practice.
Have you read?
4. Study designs and metrics need to be standardised
A major barrier to synthesising evidence on the impact of vaccines on AMR is the lack of standardisation in outcome measures, study designs, and analytical approaches.
Currently, studies use a wide variety of metrics to assess impacts on antimicrobial use and AMR genes, making it difficult to compare results or conduct meta-analyses.
The Wellcome report calls for greater alignment with emerging frameworks, such as the WHO’s considerations for research studies evaluating vaccine impact on AMR, which recommend standardised protocols and priority outcome measures. Standardisation would facilitate the aggregation of data across studies, enable more robust policy guidance and help identify where vaccines can have the greatest impact on AMR.
The report also highlights the need for international collaboration to develop and implement these standards, as well as for investments in surveillance infrastructure, particularly in resource-limited settings. By adopting common metrics and methodologies, the global health community can accelerate the generation of actionable evidence and support more effective decision-making on vaccine use for AMR control.
5. Evidence generation should prioritise pathogens and/or settings where it can most influence policy
Not all vaccines or pathogens offer the same potential for AMR impact, and resources for research and implementation are limited. The report recommends prioritising evidence generation for pathogens and contexts where AMR outcomes could be most influential in policy decisions.
By focusing on high-impact scenarios and integrating AMR considerations into vaccine investment strategies, policymakers can make more informed decisions that maximise public health benefits and help curb the spread of resistance.
This includes situations where the disease burden or vaccine efficacy is borderline, and strong evidence of AMR benefits could tip the balance in favour of vaccine introduction.
It is particularly relevant for pathogens with limited treatment options due to resistance, such as gonorrhoea or Shigella, or where vaccines could significantly reduce antibiotic use.
The report also stresses the importance of aligning research with policymaker needs, including economic analyses and syndromic data, to ensure that evidence is both relevant and actionable.
By focusing on high-impact scenarios and integrating AMR considerations into vaccine investment strategies, policymakers can make more informed decisions that maximise public health benefits and help curb the spread of resistance.
6. Policy decisions require evidence that is aligned with real-world clinical management
For evidence on vaccines and AMR to influence policy, it must be presented in ways that reflect how antibiotics are actually prescribed and how clinical care is delivered. In many settings, especially where diagnostic capacity is limited, antibiotics are prescribed based on syndromic presentation (such as fever or diarrhoea) rather than confirmed pathogen diagnosis.
The report suggests that presenting AMR data within the context of clinical syndromes, rather than only for individual pathogens, can better inform decision-making and align with existing clinical guidelines. This approach acknowledges the realities of healthcare delivery and the challenges of implementing pathogen-specific interventions in resource-limited environments.
Additionally, integrating AMR endpoints into vaccine trials and strengthening surveillance systems will help generate the kind of actionable, context-specific evidence that national and global stakeholders need. By anticipating policymaker evidence needs and designing studies accordingly, researchers and funders can reduce the risk of policy inertia or implementation delays and ensure that the full public health value of vaccination is recognised and realised.