Country readiness for COVID-19 vaccines
This article is part of a series of explainers on vaccine development and distribution. Learn more about vaccines – from how they work and how they’re made to ensuring safety and equitable access – in WHO’s Vaccines Explained series.
- 26 February 2021
- 5 min read
- by WHO
Countries are beginning to deploy COVID-19 vaccines, bringing new hope to the fight against the global pandemic. WHO, UNICEF, Gavi and many other partners are working together to support countries in preparing for COVID-19 vaccine introduction. They have provided a COVID-19 vaccine introduction toolbox that has all the resources a country needs to get ready for delivering COVID-19 vaccines. Within this toolbox, training is available for national/subnational focal points and health workers to equip them with the necessary knowledge and skills.
What is the process for countries to get COVID-19 vaccines from the COVAX Facility?
A total of 92 low- and middle-income economies will be able to access COVID-19 vaccines through the COVAX Facility Advance Market Commitment (AMC).
The COVAX AMC is the innovative financing instrument that will support the participation of 92 low- and middle-income economies in the COVAX Facility – enabling access to donor-funded doses of safe and effective COVID-19 vaccines. The AMC, combined with additional support for country readiness and delivery, will make sure the most vulnerable in all countries can be protected in the short term, regardless of income level.
These AMC92 countries must develop a COVID-19 National Deployment and Vaccination Plans (NDVPs) which is reviewed by WHO, UNICEF and other partners to help the country be sure the plan is as good as it can be. NDVPs can be submitted through the Partners Platform.
Have you read?
Once a country's NDVP is reviewed to ensure the key readiness criteria are met, they can be allocated vaccines through the COVAX Facility. Non-AMC countries are also welcome to submit their NDVP for review and receive feedback to strengthen their plan.
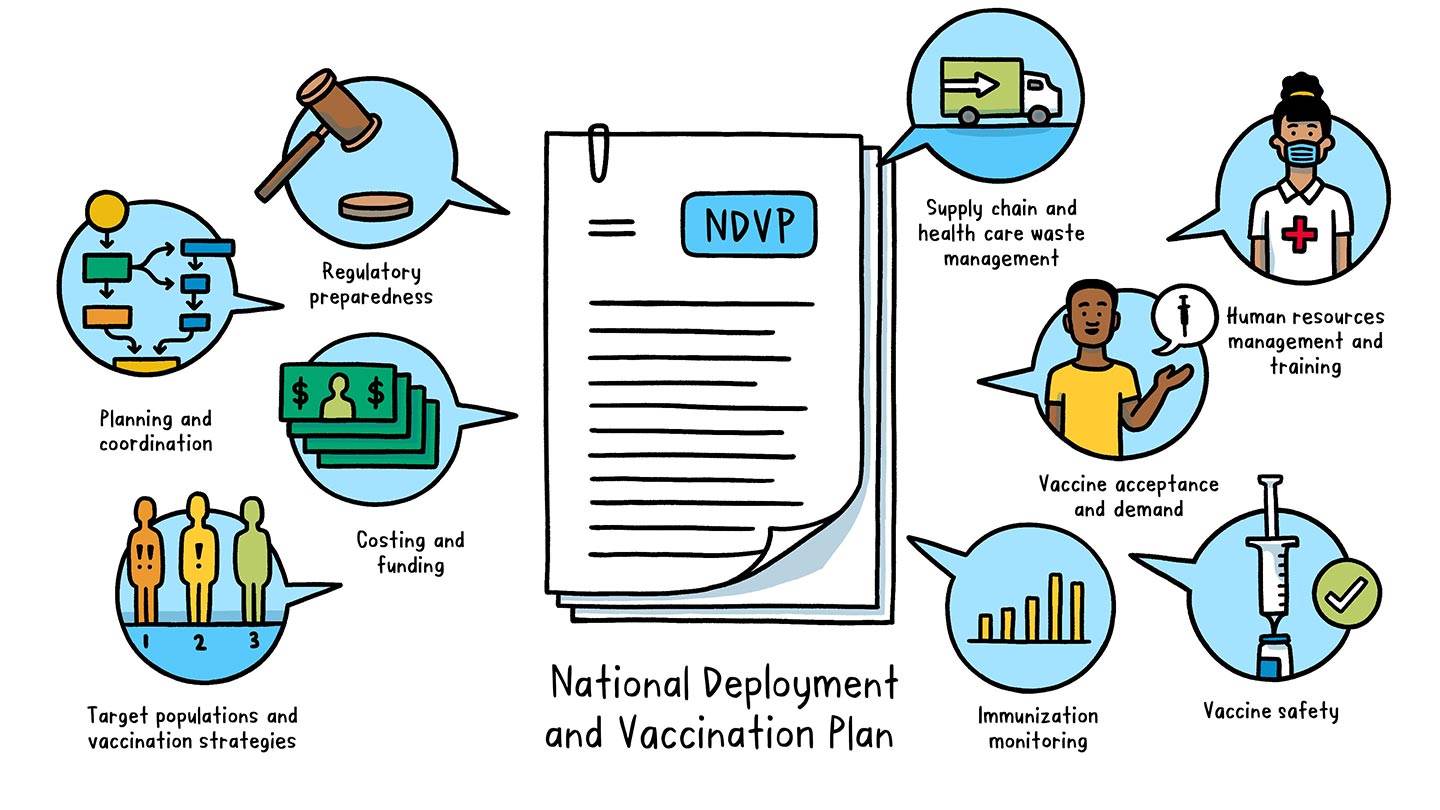
What is a National Deployment and Vaccination Plan?
A National Deployment and Vaccination Plan is an operational plan to implement and monitor COVID-19 vaccination rollout in a country. The NDVP serves as the “one-country plan” and main framework for a country’s vaccine introduction and vaccination efforts.
The NDVP outlines key aspects of readiness, including:
- Regulatory preparedness: legal requirements for importing vaccines, regulatory authorization for using vaccines, and procedures to expedite vaccine availability
- Planning and coordination: governance and management structure at both country (such as a national coordinating committee and national immunization technical advisory groups) and subnational levels to oversee the deployment, implementation and monitoring of COVID-19 vaccines
- Costing and funding: a realistic budget – including funding sources and budget gaps – for vaccine deployment and vaccination
- Target populations and vaccination strategies: order of priority for different population groups to receive vaccination and how each group will be vaccinated
- Supply chain and health care waste management: critical supply chain activities to prepare for vaccine deployment (such as cold chain assessment and secure distribution and logistics) and to ensure safe handling of health care waste
- Human resources management and training: staff requirements for the vaccine rollout and a plan for training and supervision
- Vaccine acceptance and demand: an integrated approach to achieve high uptake of COVID-19 vaccines, including strategic communication activities that promote vaccination, manage misinformation and convey risk in the event of any adverse effects
- Vaccine safety: steps to prepare for, monitor and address vaccine safety and potential adverse events and ensure injection safety
- Immunization monitoring: a strategy for collection and reporting of immunization data, disease surveillance to monitor vaccine impact, and evaluation of the overall implementation process
Each country’s NDVP should be developed through a consultative process, led by the country’s Ministry of Health and supported by other organizations, including WHO, UNICEF and other relevant partners, to fine-tune the plan until it is complete. The Country Readiness and Delivery workstream of the ACT Accelerator has issued guidance on developing a national deployment and vaccination plan for COVID-19 vaccines, which helps countries produce their plan for COVID-19 vaccine introduction.
Why should countries develop a National Deployment and Vaccination Plan?
Developing an NDVP helps countries prepare for COVID-19 vaccination, identify resource needs and streamline the process for introducing vaccines. Once an NDVP is developed, countries work on its implementation, including the detailed planning of vaccine delivery strategies, logistics, HR and funding mobilization.
How will country readiness be assessed?
A review process has been set up to provide support and feedback to countries for improving and finalizing their plans. This ensures that a country’s NDVP includes the necessary elements to rapidly deploy vaccines and implement COVID-19 vaccination, and that any recommendations for improvement are addressed.
Once an AMC92 participant submits its NDVP, a Regional Review Committee (RRC) assesses the plan and evaluates country preparedness and capacity for vaccine allocation, deployment and administration. Those participants with an NDVP that meets the standardized readiness assessment join the process for allocating COVID-19 vaccines from the COVAX Facility. Countries should take into account the recommendations from the review to further improve their preparedness for vaccine introduction.
Partner content

This article was first published by the World Health Organization on 19 February 2021.









