New study validates previous conclusions supporting a single-dose HPV vaccination schedule
Findings demonstrate non-inferiority of one dose compared to two doses of HPV vaccines to prevent cervical cancer-causing HPV infections.
- 29 January 2026
- 2 min read
- by PATH

Results from the first blinded, randomized non-inferiority trial comparing one versus two doses of HPV vaccine are now available: one dose provided similar protection to that of two doses, with observed 97% effectiveness against cancer-causing HPV infections in four trial groups totalling more than 20,000 participants measured up to five years post-vaccination.
The study reinforces the interpretation of the data used to inform the World Health Organization (WHO) 2022 alternative recommendation.
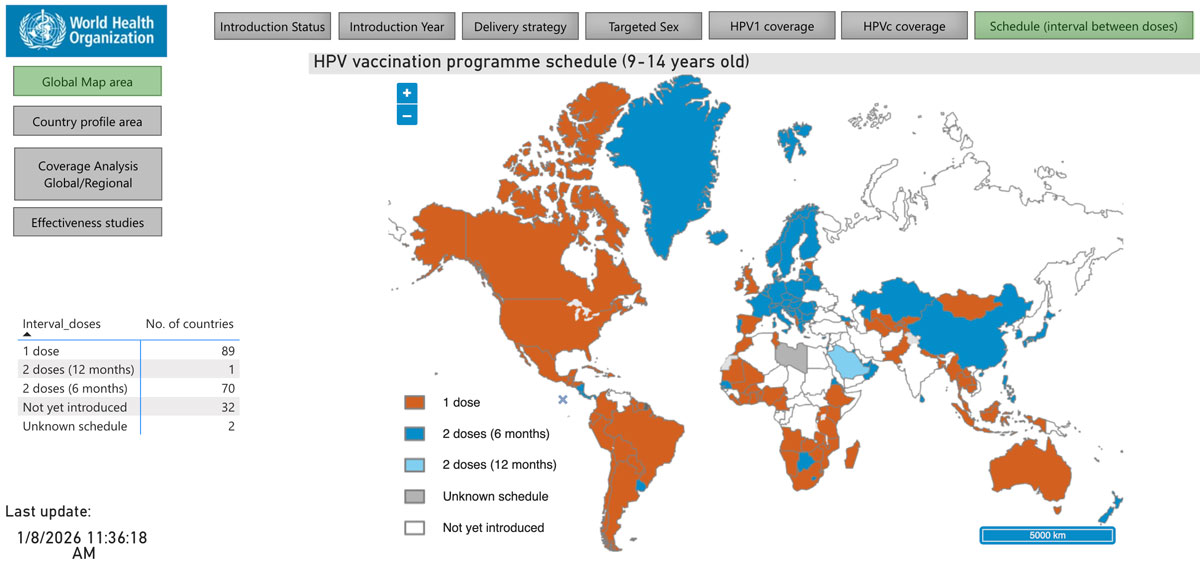
Previously, the Single-Dose HPV Vaccine Evaluation Consortium collated and evaluated the existing evidence base to inform decision-makers.
This included the randomised, controlled trial KEN-SHE that compared one dose to no vaccination; the DoRIS immunobridging analysis (comparing results from one trial to another to infer effectiveness); and single-dose HPV vaccine cohorts from high-quality observational studies.
In addition to motivating WHO to update its schedule recommendation in 2022, the strength of that evidence also convinced nearly 90 countries since to adopt a single-dose regimen.
Studies like ESCUDDO remind us that data doesn’t need to result in a breakthrough to make a difference. Validating previous conclusions with definitive clarity is a critical component of scientific research, and not always easy to obtain.
The ESCUDDO results validate the conclusions of the current evidence base because the protocol was specifically designed to assess the performance of one dose compared to two doses within the same study, resolving any lingering ambiguity in the interpretation of previous research.
For national HPV vaccination programme stakeholders that are still undecided, these results make a compelling case for making the switch. And the evidence on the durability of protection continues to build as the years go by; the longest available timeframe to date shows vaccine protection holding steady for more than a decade post-vaccination with one dose.
Studies like ESCUDDO remind us that data doesn’t need to result in a breakthrough to make a difference. Validating previous conclusions with definitive clarity is a critical component of scientific research, and not always easy to obtain.
In the case of ESCUDDO, the numbers were clear and the conclusion obvious. That reassurance is worth celebrating.