The African Vaccine Manufacturing Accelerator: what is it and why is it important?
A new innovative financing instrument, called the African Vaccine Manufacturing Accelerator (AVMA), has been approved by the Gavi Board. But why is it important for African countries to produce vaccines, how will it work, and why now?
- 7 December 2023
- 8 min read
- by Gavi Staff

A new instrument has just been approved that could help catalyse the sustainable growth of vaccine manufacturing in Africa. The African Vaccine Manufacturing Accelerator (AVMA) is designed to make up to US$ 1 billion available over the next ten years to support the sustainable growth of Africa's manufacturing base, which has the potential to not only contribute to healthy global vaccine markets, but also benefit outbreak and pandemic prevention, preparedness, response and resilience.
The case for change
The COVID-19 pandemic brought the strategic importance of access to vaccine manufacturing into the public eye and to the forefront of the minds of policymakers. The countries and regions with the strongest research, manufacturing and regulatory ecosystems were the first to access COVID-19 vaccines. Other regions, by stark contrast, were locked out of access during the early days of the pandemic, as vaccine nationalism and market failure initially held sway.
A sustainable expansion of Africa’s vaccine manufacturing capacity would have a double payoff for the continent, contributing to the growth of a high-value biotechnology sector on the continent at the same time as supporting pandemic and outbreak prevention and response.
No region felt the negative effects of COVID-19 vaccine inequity more than Africa. And no region stands to benefit more from sustainable growth in its vaccine manufacturing sector.
At present, demand for vaccines in Africa is valued at over US$ 1 billion annually, with this figure projected to grow along with the continent's population over the next several decades. Africa already accounts for around 20% of the world's population, yet the continent's vaccine industry provides only around 0.2% of global supply.
A sustainable expansion of Africa's vaccine manufacturing capacity would have a double payoff for the continent, contributing to the growth of a high-value biotechnology sector on the continent at the same time as supporting pandemic and outbreak prevention and response. At the same time, a strong vaccine manufacturing sector in Africa could benefit the overall health of vaccine markets globally.
Speaking at the annual International Conference on Public Health in Africa in November 2023, Dr Jean Kaseya, director-general of the Africa Centres for Disease Control and Prevention (Africa CDC), likened this to the continent's 'second independence'. At the same event, Gavi interim CEO David Marlow signalled Gavi's commitment to work with partners to "drive an African vaccine revolution, creating an industry that can boost economies, create jobs and help ensure that when the next pandemic hits, vaccines made in Africa are ready to protect populations".
A strong signal
Since 2001, Gavi has become one of the world's largest buyers of vaccines, working closely with African countries and manufacturers to shape the market for vaccines, and helping to expand the number of manufacturers of Gavi-supported vaccines from 5 producers to 19. So when the African Union (AU) set a bold target for African countries to produce and supply more than 60% of the continent's vaccine requirements by 2040, Gavi was a natural partner to help chart a collective path towards a sustainable manufacturing ecosystem in the region.
Since 2001, Gavi has become one of the world’s largest buyers of vaccines, working closely with African countries and manufacturers to shape the market for vaccines, and helping to expand the number of manufacturers of Gavi-supported vaccines from 5 producers to 19.
Gavi's ten-point plan for developing and strengthening vaccine manufacturing in Africa set out the key actions needed to diversify and secure vaccine supply in Africa and support of the AU's vision, including the need for Gavi to update the Alliance's market shaping model to assign greater value to vaccine supply resilience in Africa.
As part of the ten-point plan, Gavi set out the need for a new financial instrument that would send a powerful signal to global markets that Gavi will support the development of African vaccine manufacturing. And this is where AVMA comes in. AVMA aims to deliver the right balance of incentives to encourage investment in Africa, and the entrance of new African manufacturers at the scale needed to be viable on a long-term basis.
How will AVMA work?
The biotech sector in Africa is still young, and it will take time for new manufacturers to build the production scale required to be sustainable. AVMA works by offering two types of incentive payments that offset some of the initial high costs of production. Some of these payments will be higher for a subset of vaccines (the priority vaccine market group) for which there is an unmet need or a need to boost market health – or for which a manufacturer has established a prioritised vaccine technology platform in Africa that could be brought online during a pandemic response.
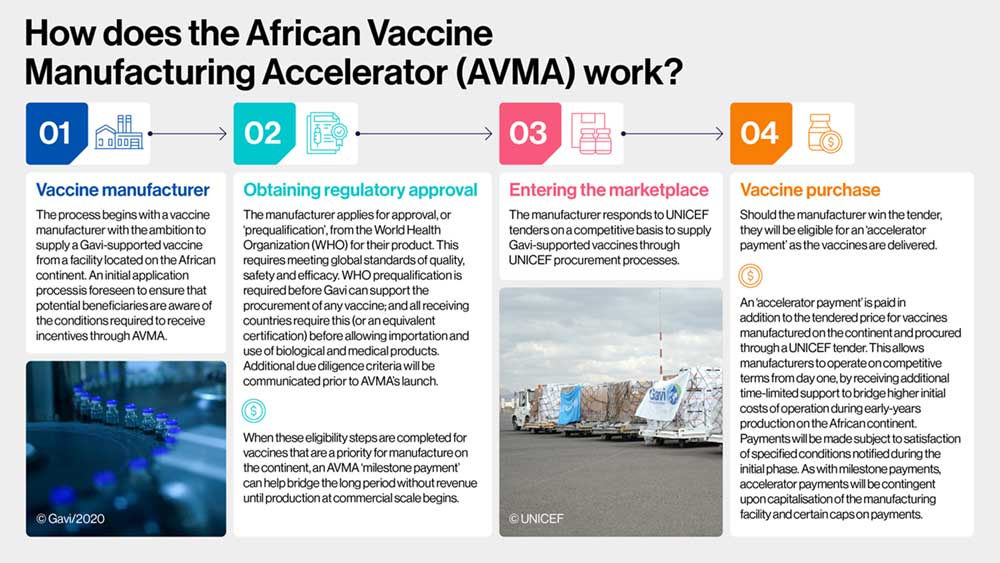
The first type of payment, known as a 'milestone payment', will be triggered when a manufacturer producing one of the vaccines included in the Gavi priority vaccine market group succeeds in obtaining WHO prequalification (PQ). PQ is a form of regulatory approval that must be obtained before a manufacturer can win a Gavi-UNICEF tender . This payment is targeted to support manufacturers to offset some of the financial burden of meeting the standards for PQ, and helps to bridge the period between this prequalification and production.
Have you read?
Milestone payments are calibrated to provide the greatest incentive to invest in modes of manufacturing most likely to support pandemic preparedness. The highest milestone payments of US$ 25 million will accrue to manufacturers that receive prequalification for vaccines produced with a 'pandemic ready' technology platform, such as the capacity to produce mRNA or viral vector vaccines. At the other end of the scale, the lowest milestone payments of US$ 10 million will accrue to manufacturers that receive PQ for 'fill and finish' manufacturing of one of the vaccines in the priority market category , whereby the final stages of production, vial filling and labelling are undertaken at an African manufacturing facility.
The second type of payment, termed an 'accelerator payment', will be paid as a per-dose 'top-up', in addition to the offered market rate manufacturers receive on winning Gavi-UNICEF tenders. These payments will be highest, at around US$ 0.50 per dose, for the end-to-end manufacture of priority market vaccines , and vaccines produced using 'pandemic ready' technology platforms. At the other end of the scale, lower tiered incentives are paid for lower-cost 'fill and finish' manufacturing, with the overall objective to incentivise a more sustainable end-to-end business model.
African vaccines for African priorities
AVMA will offer the highest per-dose incentive payments and milestone payment to manufacturers that are able to supply vaccines that have strategic importance for the African continent.
This strategic importance can derive from several factors, including the need to build supply security and resilience for vaccines against certain outbreak-prone diseases, specific opportunities for commercially sustainable new market entrants, or the need to develop and produce vaccines with product profiles optimised to meet African priorities and contexts.
The final list of priority market vaccines is designed to balance these strategic priorities, and ensure that incentives are calibrated to encourage a broad ecosystem without diluting their potential impact. The priority market vaccines are:
- Cholera vaccine
- Malaria vaccine
- Measles-rubella vaccine
- Hexavalent vaccine against diphtheria, tetanus, whole-cell pertussis [DTwP], hepatitis B, Haemophilus influenzae type b and polio
- Yellow fever vaccine
- Single-dose rotavirus vaccine in blow-fill-seal (BFS) presentation
- Ebola vaccine effective against two species of Ebolavirus, with improved thermostability at maximum -20°C
- Pneumococcal vaccine (minimum 13-valent)
With an intended capitalisation of up to US$ 1 billion, AVMA is designed to provide incentives for ten years, with various caps on incentives so that no vaccine type, or single manufacturer, is overrepresented. In addition, the availability of incentives is balanced to offer the best chance of cultivating a broad and resilient manufacturing sector.
Detailed terms and conditions to underpin AVMA and the payment of incentives will be communicated prior to the launch of the facility.
Challenges ahead
AVMA is a clear signal that the political will to achieve the African Union's vision is now being translated into purposeful and pragmatic action.
The incentive structure for the AVMA was fine-tuned during an intense 12-month period of consultation, design and modelling. This extensive testing and calibration was done with the aim of ensuring that support would maximise the sustainability of African manufacturing capacity and minimise inadvertent market distortions. But building a thriving vaccine manufacturing ecosystem in Africa will take years of steady commitment, coordination and investment.
There are numerous initiatives aimed at strengthening vaccine manufacturing in Africa. Ensuring that this multitude of initiatives and national strategies are aligned will be crucial to developing a diverse ecosystem that meets Africa's strategic economic and pandemic preparedness goals. The speed at which manufacturers can achieve regulatory prequalification will also be a crucial rate-limiting step for the expansion of the sector.
With an intended capitalisation of up to US$ 1 billion, AVMA is designed to provide incentives for ten years, with various caps on incentives so that no vaccine type, or single manufacturer, is overrepresented.
Gavi estimates that AVMA would need to disburse between US$ 750 million to US$ 1 billion to see a solid and sustainable foundation offering both resilient vaccine supply and improved pandemic response. As a minimum, the instrument aims to support at least four African vaccine manufacturers operating sustainably and at scale to win Gavi/UNICEF tenders for the production of well over 800 million vaccine doses over 10 years.
The stage is now set for African vaccine manufacturing to be transformed in the years to come.