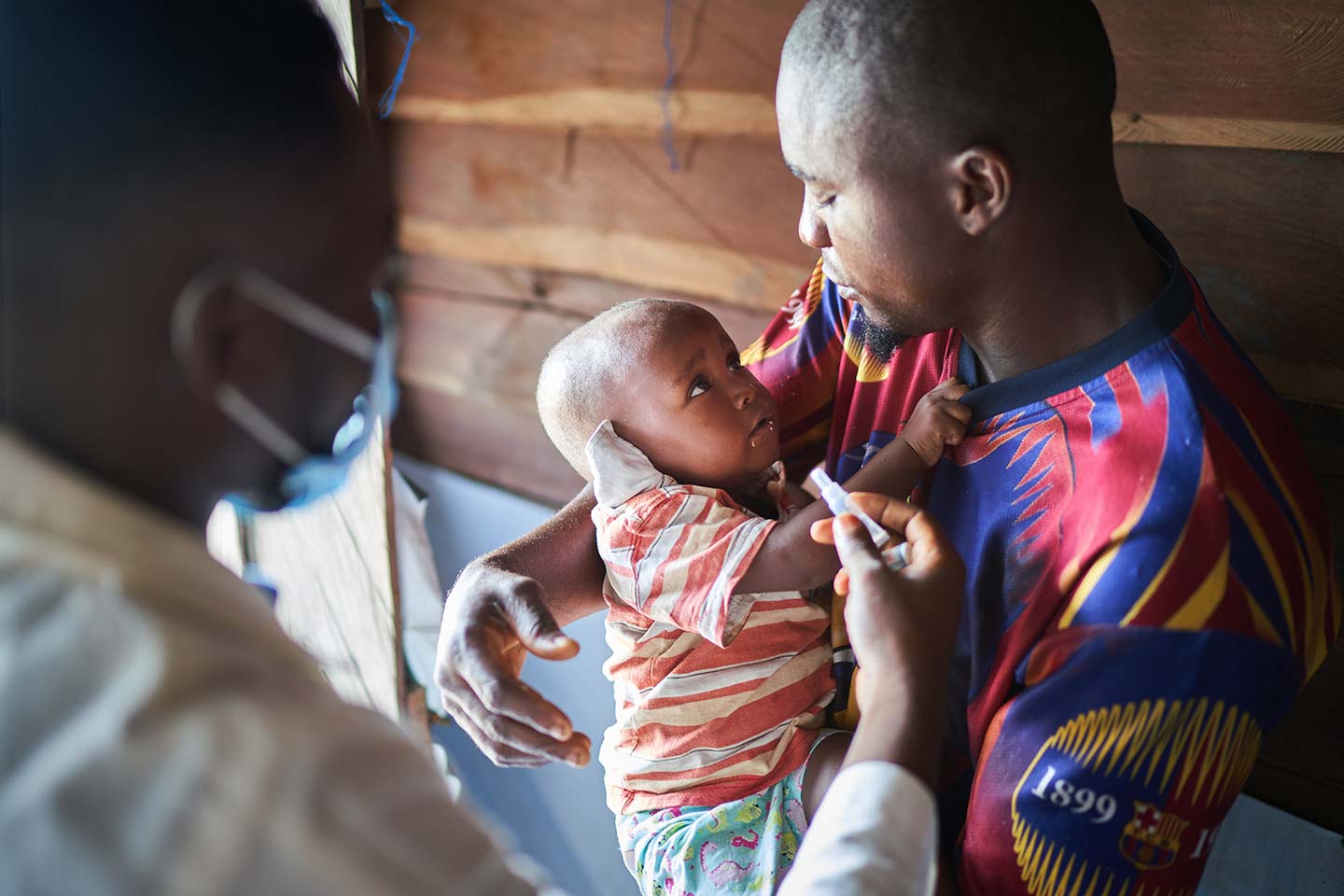
How South Africa is preparing for its COVID-19 vaccine introduction
As participating countries look towards receiving their first batch of COVID-19 vaccines through the COVAX Facility, we examine how South Africa is ramping up its readiness.
- 15 January 2021
- 8 min read
- by Tetsekela Anyiam-Osigwe

The distribution of COVID-19 vaccines through the COVAX Facility will depend on country readiness and preparedness for vaccine introduction. With Gavi and its Alliance partners providing country readiness assessment and costing budget tools, participating countries are doing their part to ensure they are adequately prepared to receive the vaccine and effectively roll it out. Among these countries is the Republic of South Africa, which is expecting to receive an allocation of COVID-19 vaccines in the coming months. Here’s how South Africa is preparing for its roll-out.
Planning and coordination
As part of any country’s readiness efforts, setting up a national coordination committee (NCC) is essential for developing an actionable national deployment and vaccination plan (NDVP) that should facilitate the swift distribution of COVID-19 vaccines once they are available.
South Africa has already established an expert Ministerial Advisory Committee and a National Technical Working Group (NTWG) to coordinate the introduction of COVID-19 vaccines. They will work in close collaboration with provincial health departments and the private health care sector. The Committee’s Covid-19 Vaccine Rollout Strategy, which intends to ensure effective vaccine delivery and administration, identifies various mechanisms to procure vaccines, including through the COVAX Facility, and multiple channels of community engagement to build trust in vaccines. Meanwhile, the NTWG has been tasked with making sure that the introduction of COVID-19 vaccines aligns closely with the strategic objectives of South Africa’s Department of Health, including creating opportunities for health system strengthening.
Expedited regulatory approvals
As COVAX aims to speed up the manufacture and distribution of COVID-19 vaccines, countries will need to have an expedited regulatory pathway in place for vaccine approvals. This could include provisions for exceptional approvals, emergency use authorisations or waivers, and fast-track mechanisms based on World Health Organization (WHO) Emergency Use Listing procedures. This will help ensure that COVID-19 vaccines can be deployed as soon as they are received.
Not all countries have robust regulatory capacity, and this is where WHO’s prequalification process is critical. As WHO Assistant Director General for Access to Medicines, Vaccines and Pharmaceuticals Dr Mariângela Simão explained in a WHO podcast last month, prequalification is important because many national regulatory authorities “don't have the technical capacity to do the full assessment.” The WHO prequalification process is “to ensure that once a vaccine is proven to be safe and effective, that actually there are no regulatory barriers for these vaccines … to be deployed at country level, no matter whether this is a very mature national regulatory authority or a less mature national regulatory authority.”
The South African Health Products Regulatory Authority (SAHPRA), which oversees regulatory approvals for the public delivery and use of vaccines, has a mechanism for expediting COVID-19 vaccine approvals. For example, SAHPRA has agreements with other agencies, including the European Medicines Agency and the US Food and Drug Administration (FDA), which allows it to use and rely on their assessment reports. Along with an enhanced flexibility in relation to labelling and packaging requirements, relying on these external assessments should reduce the timelines in the evaluation and deployment process without compromising on vaccine safety, effectiveness and quality.
Have you read?
Prioritisation, targeting and delivery strategies
Getting the first batch of vaccines is one step; the other – and more crucial step – is getting people vaccinated. This means countries, like South Africa, must make decisions regarding the target populations who will be prioritised for vaccine access and the delivery strategies to best reach them. According to the WHO, these decisions should be informed not only by local epidemiologic settings (community transmission or clusters of cases) and an assessment of risk factors, but also vaccine supply scenarios.
South Africa has opted for a phased approach to the vaccine roll-out, not only based on scientific evidence regarding infection and transmission risks, but also societal impact and principles of social solidarity. This phased approach targets approximately 67.25% of South Africa’s population in order to achieve herd immunity, according to the health minister, Dr Zweli Mkhize. Phase 1 would see the country’s estimated 1.25 million frontline health workers vaccinated primarily through facility-based delivery programmes. Essential workers, older people and anyone above the age of 18 years with co-morbidities, and in congregate settings (like prisons and care homes) – an estimated 16 million people in total – will be vaccinated in phase 2. The final phase targets an estimated 22.5 million adults over the age of 18 years.
Phases 2 and 3 will see a combination of multiple service delivery platforms, ranging from public facilities to remote community pharmacies, in order to ensure that vaccines are accessible across geographic locations. This phased roll-out will require over 6,000 full-time vaccinators, delivering more than 300,000 doses per day. Particularly during phase 3 – the largest phase – South Africa will need to carefully plan how best to optimise appropriately trained staff during a time when many could still be occupied with COVID-19 response activities (including those working on the clinical care side).
Vaccine procurement and funding
A key aspect of ensuring a successful COVID-19 vaccine roll-out is finalising the costing and budgeting for the roll-out. This includes estimating costs for the financial and human resources needed (including surge capacity requirements) to conduct vaccine deployment and administration, as well as other crucial components, like demand generation and surveillance. Alongside this, countries must also identify and secure funding, possibly in collaboration with relevant international and domestic stakeholders, in order to procure the vaccines.
South Africa has estimated that the cost of its own vaccine roll-out plan will be 20.6 billion South African rand (around US$ 1.4 billion). The National Treasury will incur most of the cost, but South Africa is largely looking towards public-private co-financing arrangements that could see medical-insurance providers contributing. Its “Committed Purchase” option agreement with the COVAX Facility – wherein South Africa made a lower upfront payment (around 15% of the total cost) with the guarantee to procure vaccine doses without opting out of specific candidates – was already financed by a Solidarity Fund. This is a platform for the public and private sectors, as well as the general public and civil society, to contribute towards various COVID-19 relief initiatives. The country’s biggest medical aid scheme, Discovery Health, has also allocated at least 7 billion rand to ensure that its members receive COVID-19 vaccines once they are made available in South Africa.
Vaccine, cold chain, and logistics
Given that the various COVID-19 vaccine candidates have different storage temperature requirements, countries need to assess which candidates are most suitable for their existing cold chain capacity. They also need to make logistics and security arrangements to safeguard vaccines during distribution.
South Africa’s current cold chain capacity is focused on safely storing vaccines in refrigerators that require storage temperatures between 2–8°C, much higher temperatures than are required for mRNA vaccines. Therefore, South Africa is looking towards vaccine candidates that are not only available and affordable, but also easily and more efficiently stored and distributed via its usual cold storage facilities.
In addition to vaccine selections that prioritise ease of introduction, South Africa is developing procedures to safeguard and track COVID-19 vaccines to avoid risk of diversion and falsification. The Ministerial Advisory Committee has outlined multiple protocols for distribution security, including vehicle tracking; data verification of volumes distributed and administered; safety and security at administration sites; as well as the monitoring of vaccine wastage.
Vaccine management, patient monitoring and surveillance
COVID-19 vaccines must also safely reach those who need them the most, and this makes monitoring and evaluation an especially vital component of any vaccine roll-out.
South Africa is in the process of developing an electronic vaccination data system (EVDS). The EVDS would provide and track vaccine information (type administered and batch number); patient information, including demographics and number of doses; safety information (possible adverse events following immunisation); and details of vaccine administration sites. As part of this monitoring system, there are plans to send reminders for a follow-up appointment to receive a second dose and to include an integrated track-and-trace system for defaulters – those who do not show up to receive their second shot. A dashboard system is also being developed to capture the reasons given for vaccine refusal.
Communication and community engagement
Even before receiving the first batch of COVID-19 vaccines, countries must start thinking about how best to enhance public understanding, and to minimise mistrust and misinformation about the vaccines. If local communities are not convinced that the vaccines can protect their health, roll-out plans are unlikely to be successful.
For its COVID-19 vaccine communication strategy, South Africa is planning a joint effort between its government agencies and civil society. This will utilise popular online social media platforms, like WhatsApp and Twitter, as well as traditional media platforms, like community radio. Building on previous effective strategies developed for other diseases, including tuberculosis and HIV, this strategy will need to engage representatives of community groups affected by COVID-19. In this way, all stakeholders would have clear guidelines to communicate scientifically sound messages to the public.