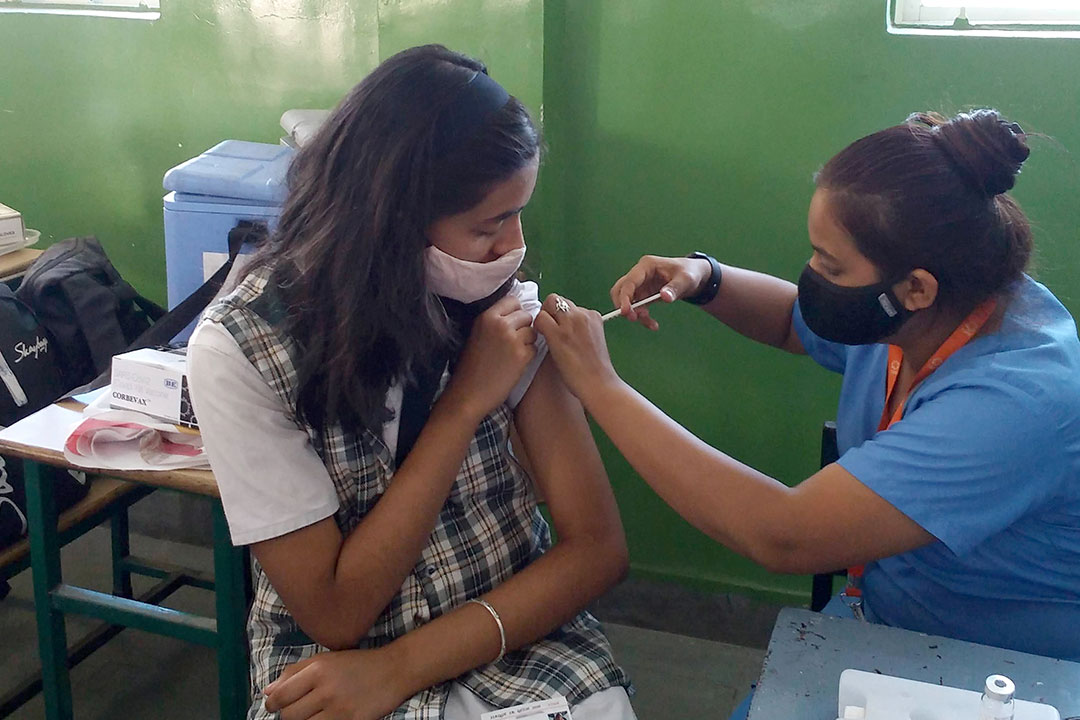Single-dose dengue vaccine ‘will help Amazon communities’
A world-first single-dose vaccine against dengue virus, manufactured and approved for use in Brazil, will benefit hard-to-reach populations.
- 4 December 2025
- 5 min read
- by SciDev.Net

A world-first single-dose vaccine against the dengue virus, manufactured and approved for use in Brazil, will especially benefit populations in hard-to-reach regions of the country such as the Amazon, say disease specialists.
The Butantan-DV vaccine, which is the first to use a single dose against the four serotypes, or strains, of dengue, was approved by Brazil’s national drug regulator last week (26 November) for use in people aged 12 to 59.
It is an important development for Brazil following its largest ever dengue epidemic, with 6.4 million cases and 5,972 deaths reported in 2024, according to the Ministry of Health.
Butantan-DV is a live, attenuated vaccine which uses weakened viruses to generate immunity without causing the disease. It was evaluated for nearly a decade in trials involving 16,000 volunteers from across Brazil.
In late-stage human trials, the vaccine showed 74.7 per cent overall efficacy and 91.6 per cent efficacy against more severe forms of the disease, according to the Butantan Institute, the public research centre that developed the vaccine.
The vaccine will be incorporated into Brazil’s national immunisation programme in early 2026, with more than 1 million doses ready to be distributed, the Institute said.
Butantan’s director, Esper Kallas called it a “powerful weapon” against the mosquito-borne disease that has plagued Brazil for decades.
Disease specialists say the one-dose vaccine will make it easier for remote communities to access. The only globally available vaccine, TAK-003 or Qdenga, requires two doses administered three months apart.
Indigenous communities will no longer have to make repeated trips to health clinics and can be fully vaccinated in one community health visit, says epidemiologist Jesem Orellana, from Brazil’s Oswaldo Cruz Foundation (Fiocruz), a leading public health research institution.
Orellana, who was not involved in developing the new vaccine, told SciDev.Net: “It is much more difficult and expensive to apply a two-dose vaccine in remote and hard-to-reach regions, such as the Amazon, crossed by rivers and with still very precarious land connections.”
60 million doses
Butantan has partnered with Chinese company WuXi Vaccines to manufacture 60 million doses over the next two years. Around half of that is expected to be delivered before the end of 2026.
The vaccination strategy and priority groups will be defined in the coming weeks by specialists from the National Immunization Program.
The new vaccine is not yet authorised for pregnant women, immunocompromised people, or older adults. Evidence of its efficacy in these populations is being analysed by Brazil’s health regulatory agency, Anvisa.

Renato Kfouri, vice president of the Brazilian Society of Immunizations, told SciDev.Net: “With this, Brazil becomes a strategic player in the international production of vaccines, and will soon be able to supply other Latin American countries also affected by the disease, such as Argentina, Peru and Colombia.”
Globally, more than 14 million dengue cases were reported in 2024, according to the World Health Organization. Of these, 12.6 million were in Latin America where more than 8,000 people died.
The Butantan Institute confirmed to SciDev.Net that it will be able to offer the new vaccine to other countries in the region, without elaborating on the timescale.
Fernanda Boulos, Butantan’s chief scientific officer, said the priority was supplying Brazil’s public health system, through the Ministry of Health.
Decade of research
Butantan-DV is a live, attenuated vaccine which uses weakened viruses to generate immunity without causing the disease. It was evaluated for nearly a decade in trials involving 16,000 volunteers from across Brazil.
Have you read?
Previous results, after two and 3.7 years of follow-up, were published in The New England Journal of Medicine and The Lancet Infectious Diseases.
The most recent data indicates that the vaccine achieved 100 per cent efficacy against hospitalisations.
A spokesperson for Butantan said these results would be published soon in the journal Nature Medicine.
Extended protection
Composed of the four serotypes of the dengue virus, Butantan-DV can be given to both people who have already had the infection and those who have never been exposed to it.
According to physician and virologist Maurício Nogueira, from the Faculty of Medicine of São José do Rio Preto, in São Paulo, this sets it apart from the other two dengue vaccines approved by Anvisa: Dengvaxia, from French vaccine maker Sanofi Pasteur and Qdenga, from Japanese pharmaceutical company Takeda.
“The first vaccine could only be administered to people who had already had dengue, which prevented its integration into the public health system,” Nogueira told SciDev.Net.
Indigenous communities will no longer have to make repeated trips to health clinics and can be fully vaccinated in one community health visit.
Takeda’s vaccine is suitable for people who have already had dengue, but its two-dose format makes adherence more difficult, says Nogueira.
“Many people who receive the first dose don’t return for the second and end up only partially protected,” he explained.
“Both doses are essential to ensure complete protection against the pathogen.”
Mosquito control
Disease specialists warn, however, that the vaccine alone will not eradicate the disease and stress the continued need for other control measures.
Climate change and urbanisation have produced increasingly favourable conditions for the Aedes aegypti mosquito, the main disease vector.
“The vaccine is important, but it doesn’t mean we should neglect mosquito control,” said Orellana, citing the risk of other mosquito-borne diseases such as chikungunya and yellow fever.
“Control remains essential, surveillance is fundamental, and it is necessary to invest in measures that reduce the vector’s reproductive capacity,” he added.
This article was produced by SciDev.Net’s Latin America and Caribbean desk.