Not all vaccines fight infections. An experimental fentanyl shot shows why
The fentanyl vaccine doesn’t aim to stop an infection, and it’s not the first like this. From toxoid vaccines to experimental shots for cancer and addiction, vaccines are gradually expanding their mission.
- 22 January 2026
- 5 min read
- by Linda Geddes
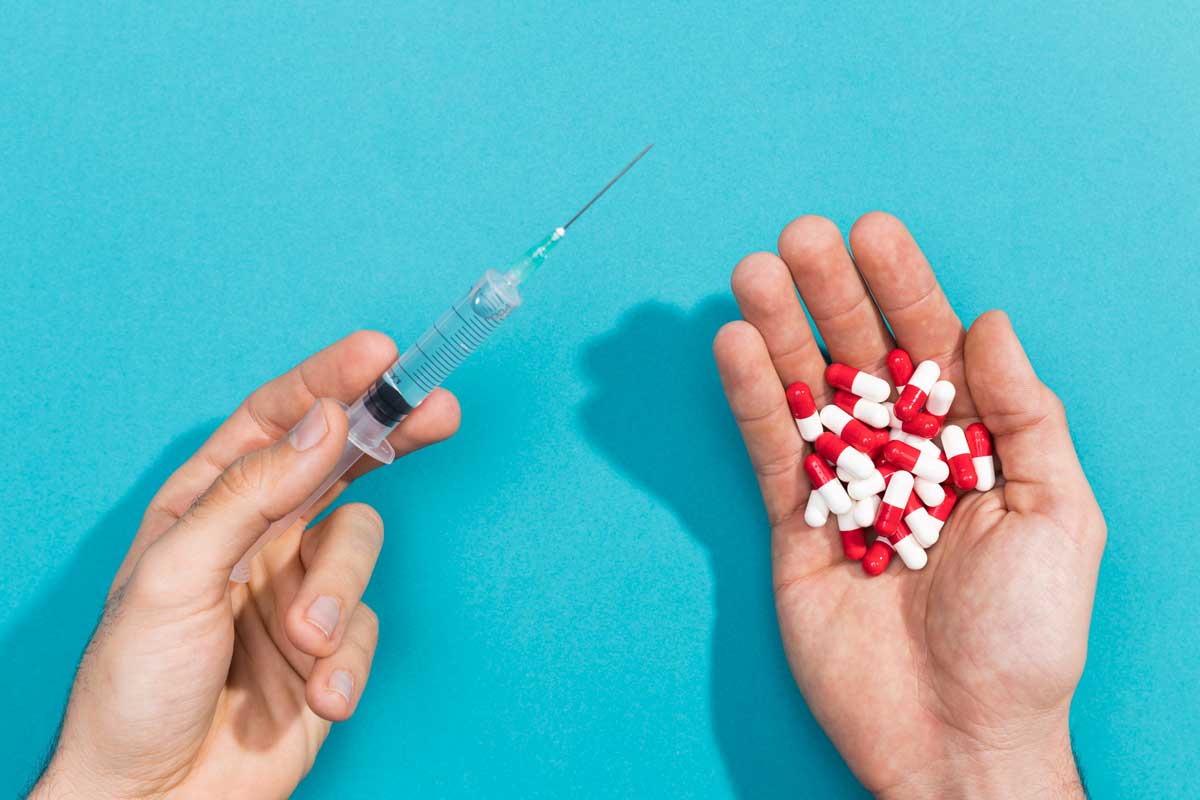
A vaccine against fentanyl may sound odd at first. Fentanyl isn’t a virus, bacterium, or parasite – it’s a synthetic opioid painkiller.
But an experimental vaccine now entering human trials works by training the immune system to recognise fentanyl and intercept it before it reaches the brain, preventing overdoses and potentially providing a proactive treatment for opioid use disorder.
While unusual, the approach is not unprecedented. The fentanyl shot belongs to a small but growing class of vaccines that aim to neutralise harmful molecules rather than disease-causing microbes.
How does the experimental fentanyl vaccine work?
Vaccines work by training the immune system to recognise a specific target and respond more quickly the next time it appears.
Traditional vaccines introduce a harmless version or fragment of a virus or bacterium, prompting immune cells to produce antibodies and memory cells that can rapidly neutralise the real pathogen should they encounter it in the future.
The experimental vaccine links fentanyl-like molecules to a larger carrier protein, allowing the immune system to recognise the drug and produce fentanyl-binding antibodies.
Fentanyl, however, is not a pathogen. It is a powerful synthetic opioid painkiller, legally used in medicine but also produced illicitly and mixed into street drugs. Because fentanyl is extremely potent, even tiny amounts can suppress breathing and lead to fatal overdose.
The receptors that fentanyl binds to are found in the brain, which only allows certain molecules in, and blocks entry of large proteins and most complex compounds.
The experimental vaccine links fentanyl-like molecules to a larger carrier protein, allowing the immune system to recognise the drug and produce fentanyl-binding antibodies.
These antibody-drug complexes are too large to enter the brain, blunting fentanyl’s effects and reducing overdose risk rather than preventing drug use. Early human trials, expected to begin in 2026, will focus on safety and whether the vaccine reliably triggers an immune response.
Which other vaccines target molecules rather than microbes?
The approach taken by the developers of the experimental fentanyl vaccine is clever, but not entirely new.
Toxoid vaccines are a longstanding example of immunisation that targets a harmful molecule rather than a microbe – specifically the toxins responsible for diseases such as tetanus and diphtheria, rather than the bacteria that produce them.
In these infections, the bacteria – Clostridium tetani and Corynebacterium diphtheriae – tend to remain localised, in a wound or on the surface of the throat, while the toxins they release spread through the body, damaging nerves or cells.
Toxoid vaccines use chemically inactivated versions of these toxins to train the immune system to produce antibodies that neutralise the toxin as soon as it is released, so it can no longer cause harm.
The bacteria may still be present briefly, but without their toxins they are far less dangerous and can be eliminated by normal immune defences or antibiotics.
While licensed toxoid vaccines are limited to toxins produced by pathogens, they demonstrate a key principle: the immune system can be trained to disarm a dangerous molecule rather than attack a microbe.
Experimental anti-drug vaccines and some cancer vaccines build on that same idea.
Are other anti-drug vaccines being developed?
Researchers have been exploring vaccines to reduce the effects of addictive drugs by training the immune system to recognise the drug itself.
These experimental vaccines encourage the body to produce antibodies that bind to substances such as nicotine, cocaine, opioids or methamphetamine in the bloodstream, making it harder for them to reach the brain and produce their usual effects.
Studies in animals and early human trials have shown that this approach can work biologically, with vaccines capable of generating antibodies that bind addictive drugs in the bloodstream.
Most current cancer vaccine research focuses on therapeutic vaccines designed to treat existing cancers by stimulating immune responses against tumour-associated or tumour-specific antigens.
However, results in people have been inconsistent. Some individuals produce strong antibody responses, while others do not, and antibody levels often decline over time without repeated booster shots.
It also remains unclear whether such vaccines can reliably change long-term drug use. For example, although a cocaine vaccine known as TA-CD advanced to Phase 3 trials and was found to be safe, it did not significantly reduce cocaine use overall, even though some participants developed high antibody levels.
An experimental nicotine vaccine, NicVAX, also reached Phase 3 trials but failed to improve smoking quit rates compared with placebo.
Because of these limitations, no anti-drug vaccines have yet been approved for clinical use.
Have you read?
How do cancer vaccines work?
Researchers are also applying the same basic idea in the development of cancer vaccines, though the aim here is to help the body identify and attack cancer cells rather than neutralise a drug or toxin.
This approach is distinct from licensed vaccines against human papillomavirus (HPV) and hepatitis B virus, which prevent certain cancers by blocking viral infection.
Instead, most current cancer vaccine research focuses on therapeutic vaccines designed to treat existing cancers by stimulating immune responses against tumour-associated or tumour-specific antigens expressed by cancer cells, such as proteins involved in tumour growth or mutations unique to an individual’s cancer.
Many of these vaccines – including peptide-based, mRNA, and ‘neoantigen’ vaccines designed uniquely for an individual patient’s tumour – are now in early- and mid-stage clinical trials, where they have been shown to be generally safe and capable of triggering immune responses.
A small number of candidates have progressed further in combination with other immunotherapies, such as checkpoint inhibitor drugs.
However, consistently translating these immune responses into clear, long-lasting clinical benefits across different cancers remains a major challenge, and most cancer vaccines are still experimental rather than part of routine treatment.