The Vaccine Innovation Prioritisation Strategy (VIPS) represents an unprecedented three-year collaboration – known as the VIPS Alliance – between the Gavi Secretariat, World Health Organization (WHO), Bill & Melinda Gates Foundation, UNICEF and PATH. Its purpose is to develop a single integrated framework to evaluate, prioritise and drive forward vaccine product innovations. |  |
An Alliance initiative

| 
| 
| 
| 
|
Addressing immunisation barriers and achieving immunisation coverage and equity goals requires innovative approaches. Vaccine product innovations simplify logistics, increase the acceptability and safety of immunisation, minimise missed opportunities, and facilitate outreach. There is increasing recognition of the need to employ targeted solutions to extend vaccine access to communities missed by immunisation services.
The VIPS process involved in-depth research, stakeholder consultations, and development and application of a methodology capable of evaluating a variety of technologies at different stages of product development. The work required understanding countries’ needs to consider the expected financial and non-financial impacts of innovations; developing common principles across the Alliance to assess the long-term benefits of product innovations; and convening a platform of stakeholders to articulate a clear and aligned perspective on priority innovations.
Prioritising innovations in vaccine products allows VIPS to provide greater clarity to manufacturers and partners, informing and influencing investment decisions. VIPS outcomes also help to mobilise key decision-makers and funders, and chart a strategic pathway forward for the prioritised innovations.
More details can be found on the methodology, process and outcomes; scope of innovations assessed within VIPS; and VIPS Steering Committee here:
- Download: VIPS methodology and outcomes summary
- Download: VIPS Steering Committee members
Final VIPS prioritisation
The VIPS process concluded in May 2020 with a decision to prioritise three innovations/approaches in which the Alliance will engage to advance development, policy and access:
- upstream novel delivery device – microarray patches (MAPs);
- combined formulation, regulatory and novel programmatic approach to vaccine management – heat-stable and controlled temperature chain (CTC)-qualified vaccines; and
- an implementation/system innovation – barcodes on primary packaging
The next step for the VIPS Alliance is to define end-to-end strategies for the three prioritised innovations, including developing action plans to accelerate uptake and impact.
VIPS Alliance 2021–2025 MAPs action plan
The VIPS Alliance’s long-term vision for microarray patches (MAPs) is to implement MAP products for priority vaccines. This will help to overcome immunisation barriers, ensuring more equitable access to vaccines in lower-income countries and contribute to global health security.
To achieve this long-term vision, the VIPS Alliance has developed an end-to-end five-year action plan for vaccine MAPs that:
- identifies activities needed to accelerate development and future uptake of vaccine MAP products for low- and middle-income country (LMIC) use; and
- aspires to advocate for vaccine MAPs and attract the interest of other global health partners and funders.
More details on the five measurable target outcomes and underlying activities identified can be found in the public summary of the VIPS Alliance Action Plan for MAPs (also linked below). The public summary is a condensed version including key background on MAPs and the list of target outcomes and activities. A longer version of the action plan is available upon request.
- Download: VIPS Alliance Action Plan for MAPS – Public Summary
- Related article: 11 needle-free vaccines that could transform global immunisation
Identification of priority vaccines for microarray patches (MAPs)
After VIPS prioritised innovations, consultations with manufacturers and developers revealed the need for clearer guidance on priority vaccines for MAPs. Hence, VIPS conducted an exercise to identify vaccines relevant to lower-income countries for which MAPs would be most valuable from a programmatic perspective, as well as technically feasible.
This resulted in a priority list of 11 target vaccines for MAPs divided into two priority groups. The methodology and outcomes of the exercise have been validated through stakeholder and expert consultations.
| Priority list of vaccine targets for MAPs | |
|---|---|
Priority group 1 | Hepatitis B |
| Measles-rubella (MR)/measles, mumps and rubella (MMR) | |
| Human papillomavirus (HPV) | |
| Rabies | |
| Yellow fever | |
| Influenza virus: seasonal and pandemic | |
| COVID-19 | |
Priority group 2 | Group B streptococcus (GBS), S agalactiae |
| Meningococcal A,C,W,Y,(X) | |
| Salmonella Typhi | |
| Streptococcus pneumoniae |
For two of the targets in the priority list, measles-rubella (MR) and typhoid conjugate vaccine (TCV), the WHO Full Value of Vaccines Assessments (FVVA) Assessment of MR-MAPs
An initial FVVA consisting of a set of qualitative and quantitative analyses was conducted to: (i) assess global need for improved measles-rubella (MR) vaccines and how MR-MAPs could address MR problem statements; (ii) estimate costs and benefits of MR-MAPs; (iii) identify the best pathway from development to delivery; and (iv) identify areas of need where stakeholder intervention can be helpful.
These analyses showed the potential of MR-MAPs as a critical innovation to overcome the limitations of traditional injectable vaccines, especially at the last mile. Measles vaccination remains a high priority, with more than 140,000 annual deaths caused by measles worldwide.
The FVVA shows that if MR-MAPs were broadly deployed between 2030 (current assumption for first licensure and WHO prequalification) and 2040, they could reach an additional 80 million children compared to traditional injectable vaccines. This would prevent up to 37 million measles cases and 400,000 measles-related deaths.
MR-MAPs are expected to have an expanded reach compared to traditional injectable vaccines because they are easier to use, so they could be delivered by community health care workers. To address critical challenges in MR immunisation efforts, MR-MAPS will be a single-dose presentation (saving time and effort compared with multiple doses) and also have improved thermostability.
Full Value of Vaccine Assessment of TCV-MAPs
The FVVA for typhoid conjugate vaccine microarray patches (TCV-MAPs) developed as a comprehensive framework for evaluating TCV-MAPs. The assessment analysed the health, economic and societal impacts of the vaccines to assess their potential value in comparison to existing vaccines. This analysis aims to optimise typhoid prevention and response efforts, particularly in lower-income countries.
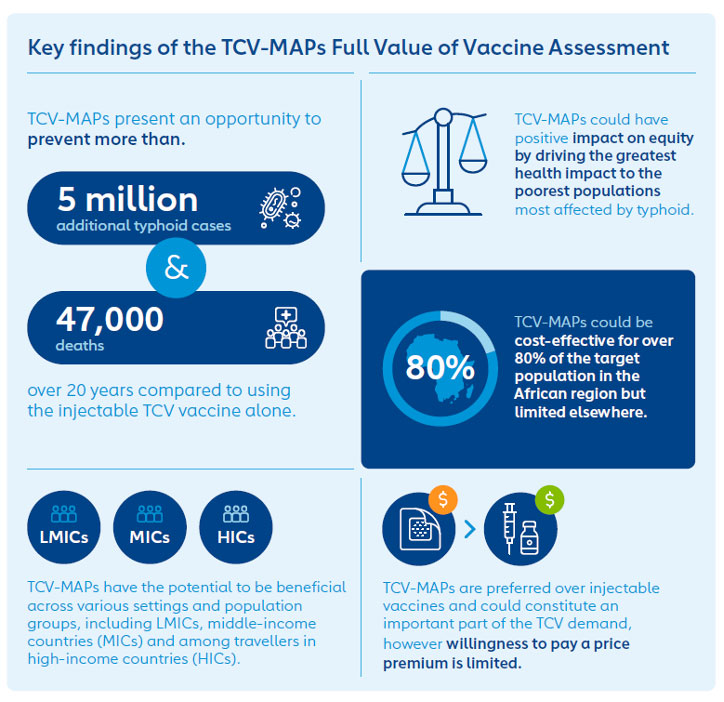
In endemic countries, TCV-MAPs could be administered in various settings, including fixed posts, outreach programmes and mobile units. TCV-MAPs could be used in routine immunisation to reach children aged under 2 years, as well as children aged 2 to 15 years in all settings for catch-up campaigns during the initial introduction of TCV. In non-endemic settings, where typhoid is rare, it still poses a significant health risk for international travellers. New vaccine presentations such as TCV-MAPs could also be beneficial in high-resource settings by improving uptake of TCV among travellers and military personnel.
Globally, the introduction of TCV-MAPs could reduce the burden of typhoid fever, with the potential to prevent over 5 million additional typhoid cases and 47,000 deaths over a span of 20 years, primarily in the African region. This represents 2% and 3% reductions in cases and deathsrespectively, compared to injectable TCV.
TCV-MAPs are expected to have a positive impact on immunisation equity. The greatest health benefits are anticipated among the lowest-income populations, who are most vulnerable to enteric diseases (i.e. illnesses related to the intestinal tract caused by lack of access to clean water and sanitation) and who may be missed by traditional immunisation efforts.
- Download: TCV-MAPs FVVA Factsheet
- Download: TCV-MAPs FVVA Report
VIPS Alliance 2021–2025 action plans for heat-stable and controlled temperature chain-qualified vaccines
Controlled temperature chain (CTC) is a designation meaning that a vaccine can be used outside of the cold chain. CTC use of vaccines allows a single excursion of the vaccine into ambient temperatures not exceeding 40°C for a minimum of three days, just prior to administration. The VIPS Alliance’s long-term vision for CTC-qualified vaccines is to enable qualification and CTC use of vaccines. It also aims to ensure that CTC-qualified vaccines will be used in countries to help overcome barriers to immunisation and ensure equitable access to vaccines.
In the short term, the focus of the CTC Action Plan is to:
- contribute to generation of evidence on the impact of CTC-qualified vaccines through robust research studies that address key barriers to CTC; and
- in parallel, accelerate the most promising opportunities to facilitate the availability and uptake of CTC for priority vaccines in lower-income countries.
Activities to generate evidence on CTC are already underway, with results expected in 2025. The VIPS Action Plan will be refined after the results of these studies have been assessed and published. More information on the research studies and CTC Action Plan is available upon request.
The VIPS strategy on improved vaccine heat stability is being defined and will be covered in a separate action plan.
Identification of priority vaccines for CTC use
After VIPS prioritised innovations, consultations with manufacturers and developers revealed the need for clearer guidance on priority vaccines for CTC use. Hence, VIPS conducted an exercise to identify vaccines relevant to lower-income countries for which CTC use would be most valuable from a programmatic perspective, as well as being technically feasible.
This resulted in a priority list of eight target vaccines for CTC use. The methodology and outcomes of the exercise have been validated through stakeholder and expert consultations.
For CTC use, the outcomes of this exercise will be complemented by the studies that VIPS is undertaking to measure the impact of CTC and to understand the use cases and contexts where it adds value. The CTC prioritisation may thus be revised after assessment of these results.
| Priority list of vaccine targets for CTC use |
|---|
| Tetanus-diphtheria (reduced D antigen for adults/adolescents) |
| Hepatitis B birth dose |
| Human papillomavirus (HPV) |
| Measles-rubella (MR) microarray patch (MAP) |
| Meningococcal A, C, W, Y (X) |
| Oral cholera vaccine (OCV) |
| COVID-19 |
| Typhoid conjugate vaccine (TCV) |
VIPS Alliance roadmap for barcodes implementation on vaccine products in LMIC
The VIPS Alliance’s long-term vision for barcodes implementation is to support equitable vaccine coverage in low- and middle-income countries (LMICs) by improving programme efficiency and enhancing health system digitalisation. Use of barcodes on vaccine products can:
- improve availability of vaccines of assured quality and reduce wastage at all levels through automation of stock management, improving data quality, vaccine forecasting and optimising workflows;
- reduce the risk of falsified vaccines and diversion outside of vaccine supply chains; and
- improve patient safety by facilitating cases of product recall if needed.
To achieve this long-term vision, the VIPS Alliance has developed an end-to-end action plan that is a first step towards a coordinated plan to advance and increase barcodes use among all stakeholders working on vaccine traceability, to generate awareness; and to ensure country ownership and funding. Further work is needed to socialise this work across relevant stakeholders.
More details on the five measurable target outcomes and underlying activities can be found in the public summary of the VIPS Alliance Roadmap for Barcodes (see document below) and in supporting annex documents, including background information and findings from VIPS consultations that informed the roadmap. For more information, please contact the VIPS team at info@gavi.org, with the words “VIPS team” in the Subject line of your email.
Download: VIPS Alliance Roadmap for Barcodes Implementation on Vaccine Products in LMICS – Public Summary
Supporting documents:
- Download: Annex A: Background on barcodes, traceability initiatives & current guidelines
- Download: Annex B: Detailed findings from VIPS country & manufacturer consultations on barcodes


