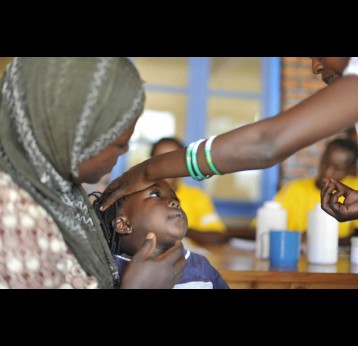
Gavi undertakes centralised and decentralised evaluations, which include the following types of evaluations corresponding to Gavi functions and programmes1:
- Strategic evaluations: Assess the quality of design, extent of implementation, results and sustainability of specific strategies, policies, processes, frameworks and models adopted by Gavi. Strategic evaluations are in most cases centralised.
- Thematic evaluations: Evaluation of a selection of interventions, all of which address a specific priority that cuts across countries, regions, and sectors. Thematic evaluations are in most cases centralised.
- Country and Programme evaluations: Evaluation to assess design, programmatic results and sustainability of Gavi supported programmes in specific countries. Country and Programme evaluations are in most cases decentralised.2
The Evaluation Advisory Committee (EAC) established by the Board of the Gavi Alliance supports the Board in fulfilling its oversight responsibilities in respect to the management of Gavi’s evaluation activities (as defined in the Gavi Alliance Evaluation Policy).
Centralised Evaluations (with EAC oversight)
Evaluation of Gavi’s contribution to reaching zero-dose children and missed communities Year 2 annual report
Gavi, the Vaccine Alliance commissioned Ipsos to conduct an independent evaluation of Gavi’s contribution to reaching zero-dose children and missed communities.
Evaluation of Gavi’s contribution to reaching zero-dose children and missed communities Year 1 annual report
Gavi, the Vaccine Alliance appointed Ipsos to conduct a multi-year evaluation of Gavi’s contribution to reaching zero-dose children and missed communities.
Mid-term evaluation of Gavi’s 2021–2025 strategy
Gavi, the Vaccine Alliance appointed Euro Health Group (EHG) to conduct a mid-term evaluation on the implementation of Gavi’s 2021–2025 strategy (Gavi 5.0/5.1).
Evaluation of the operationalisation of Gavi’s strategy through policies, programmatic guidance and use of funding levers
Gavi, the Vaccine Alliance appointed Euro Health Group (EHG) to conduct an evaluation of the operationalisation of Gavi’s strategy through policies, programmatic guidance and use of funding levers.
COVAX Facility and COVAX Advance Market Commitment (AMC) Formative Review and Baseline Study
Gavi, the Vaccine Alliance appointed Itad to conduct an evaluability assessment and evaluation design study, and a baseline study of the COVAX Facility and COVAX Advance Market Commitment (AMC).
Evaluation of Gavi’s initial response to COVID-19
Gavi, the Vaccine Alliance appointed Euro Health Group (EHG) to conduct an evaluation of Gavi’s initial response to COVID-19.
Evaluation of the Cold Chain Equipment Optimization Platform
Gavi, the Vaccine Alliance commissioned a prospective independent, multi-year evaluation of its CCEOP investment to a coalition led by JSI Research & Training Institute, Inc.(JSI) with Stat View International (Guinea), JaRco Consulting (Kenya…
Gavi Pneumococcal Conjugate Vaccine Advance Market Commitment pilot: 2nd Outcomes and Impact Evaluation
Gavi, the Vaccine Alliance, commissioned an independent outcome and impact evaluation of Gavi’s Pneumococcal Conjugate Vaccine Advance Market Commitment (PCV AMC) led by Dalberg.
Evaluation of Gavi's Fragility, Emergencies and Refugees policy
Gavi, the Vaccine Alliance, appointed HERA to conduct an evaluation of Gavi’s Fragility, Emergencies and Refugees (FER) Policy (2017).
Gavi’s COVAX Facility and COVAX AMC Evaluability Assessment and Evaluation Design Study
Gavi, the Vaccine Alliance, appointed Itad to conduct an evaluability assessment and evaluation design study for the COVAX Facility and COVAX AMC.
Evaluation of Gavi’s Private Sector Engagement Approach 2016-2020
Gavi, the Vaccine Alliance, appointed Mott MacDonald to conduct an evaluation of Gavi’s Private Sector Engagement Approach (PSEA) 2016-2020.
Evaluation of the Gavi Supply and Procurement Strategy, 2016-2020
This report presents the findings from the ‘Evaluation of the Gavi Supply and Procurement Strategy, 2016-2020’. The evaluation was conducted by CEPA in 2020, with the purpose to generate evidenced-based data and learning to inform the Gavi Supply…
Co-financing, eligibility and transition policies evaluation 2019
Gavi, the Vaccine Alliance, appointed Cambridge Economic Policy Associates (CEPA) to conduct an evaluation of Gavi’s Eligibility and Transition and Co-financing Policies.
Measles campaigns in Nigeria
This report presents the main findings of the evaluation of Gavi supported measles supplementary immunisation activities in Nigeria.
Evaluation of Gavi support to CSOs
This report presents the main findings from an evaluation of Gavi’s support to civil society organisations (CSOs) during the period 2011 to 2017. The evaluation was conducted by Itad in 2018.
Gender policy evaluation 2019
This report presents the findings from the evaluation of Gavi’s 2013 Gender Policy. The evaluation was conducted by Itad in 2018-2019.
Health system strengthening tracking study
The 2008 HSS tracking study is complementary to the HSS evaluation, providing a more in depth country perspective that helped highlight implementation issues in 6 countries.
Full country evaluations
Gavi, the Vaccine Alliance launched a prospective evaluation to collect real-time data on immunisation programmes, vaccine-related issues and the contribution of Alliance support in four countries.
Review of Gavi’s Performance-Based Funding component of its Health System Strengthening support
In 2018 the Gavi Evaluation Unit commissioned an independent review of Gavi’s Performance Based Funding (PBF) component of its Health System Strengthening (HSS) support to countries. This review was conducted by Duke University between April and…
Technical assistance through the Partners Engagement Framework evaluation
An evaluation is currently under way to assess the relevance, effectiveness and efficiency of technical assistance to countries through Gavi’s partners’ engagement framework (PEF).
Gavi support to Albania evaluation
This report presents the main findings from an evaluation of Gavi’s support to Albania and its transition after funding ended. The evaluation was conducted by the Curatio International Foundation in 2015.
Health system strengthening evaluations 2013-2015
Gavi’s health system strengthening (HSS) support is regularly evaluated to provide valuable insights into the HSS programme.
Gavi support to Bosnia and Herzegovina evaluation
This report presents the lessons learnt and recommendations from an evaluation of Gavi’s support to Bosnia and Herzegovina and its transition after funding ended. The evaluation was conducted by the Curatio International Foundation in 2014.
Co-financing Policy Evaluation
This report presents the lessons learnt and recommendations from an independent evaluation of the Gavi co-financing policy conducted by the Norwegian Institute of Public Health in 2014.
Evaluation of the Gavi-Government of China Hepatitis B vaccination programme
This report presents the findings and lessons from an evaluation of the Gavi-Government of China Hepatitis B vaccination programme. The evaluation was conducted in 2012 by Abt Associates.
Gender policy evaluation
This report presents the findings and lessons from an evaluation of the Gavi Gender Policy. The evaluation was conducted in 2012 by ICF Macro, Inc.
Pneumococcal AMC process and design
This report presents the findings of the Process and Design Evaluation for the Pilot Advance Market Commitment (AMC) for Pneumococcal Vaccines. This evaluation was conducted in 2012 by Dalberg Global Development Advisors.
Pneumococcal AMC pilot outcomes and impact evaluation
This report presents the findings of the Outcomes and Impact evaluation for the Pilot Advance Market Commitment (AMC) for Pneumococcal Vaccines. This evaluation was conducted in 2015 by The Boston Consulting Group.
Evaluation of Gavi support to CSO 2012
This report presents the findings and lessons from a review of Gavi’s support to civil society organisations undertaken by Cambridge Economic Policy Associates.
Accelerated Vaccine Introduction project review
Lessons learnt from the Accelerated Vaccine Introduction (AVI) Project (published 2012).
International Finance Facility for Immunisation evaluation
The June 2011 evaluation of the International Finance Facility for Immunisation (IFFIm) assesses whether the IFF concept is proven and whether the IFFIm pilot has worked.
Baseline study for AMC
The Baseline Study for the pneumococcal vaccine Advance Market Commitment establishes the environment prior to the AMC as a basis for future evaluations.
Injection safety support evaluation
Gavi commissions 2008 report to analyse the sustainability of its Injection Safety Support.
Immunisation services support evaluation
External review of effectiveness of Gavi's ground-breaking Immunisation Services Support over its first five years (2000-2005).
Gavi second evaluation report
The Gavi second evaluation report sets out the main findings of an independent assessment of the Vaccine Alliance's achievements in Phase II (2007-2010).
Gavi first evaluation report
The Gavi first evaluation report sets out the main findings of an independent assessment of the Vaccine Alliance's achievements in Phase I (2000-2005).
ADIPs and Hib Initiative Evaluation
2007 evaluation of Gavi's efforts to introduce new vaccines via the Accelerated Development and Introduction Plans and the Hib Initiative.
Health system strengthening review
The 2009 review of Gavi's health system strengthening support includes 21 country case studies.
Decentralised Evaluations
Synthesis Report of PEF-TCA Assessments in High-impact Countries
This synthesis report brings together findings from the rapid country assessments of Partners’ Engagement Framework Targeted Country Assistance (PEF-TCA) in three of Gavi’s High Impact countries (HICs): Democratic Republic of the Congo, Ethiopia and Nigeria.
Evaluating the effective use of DHIS2 for improving planning and implementing HIV, TB, Malaria, and Immunisation programs in Uganda, Bangladesh and Mali
Vaccine Alliance, and The Global Fund to Fight AIDS, Tuberculosis and Malaria commissioned a three-country retrospective evaluation to examine the adoption and effectiveness of DHIS2 in supporting HIV, TB, malaria, and immunisation programmes across Uganda, Bangladesh, and Mali. The study provides key recommendations to enhance the scalability, sustainability, and equity of DHIS2 implementation.
Gavi PEF TCA country assessments: meta-review
Gavi, the Vaccine Alliance commissioned IOD PARC to conduct an evaluability assessment of the Targeted Country Assistance (TCA) provided through Gavi’s Partners’ Engagement Framework (PEF). The assessment consisted of a meta-review of PEF TCA in six countries and aimed to generate recommendations to improve the performance and evaluability of the PEF TCA system for Gavi 5.0.
Evaluation Report of the Toyota Vaccine Land Cruiser
This report presents an evaluation of the Vaccine Land Cruiser’s performance in Niger, Burkina Faso, Senegal, and South Sudan, focusing on its ability to enhance access, efficiency, and safety in vaccine distribution.
Evaluating the effectiveness of Zindagi Mehfooz Electronic Immunization Registry
The Zindagi Mehfooz (Safe Life) Electronic Immunization Registry (ZMEIR) in Sindh Province, Pakistan is a comprehensive suite of digital health interventions which aims to improve equitable access, timeliness, and coverage of child immunizations through a smartphone-based application (app) for vaccinators, web-based dashboards for supervisors and managers, and a text message reminder system and call centre for caregivers.
Investigating the use of digital solutions in the COVID-19 pandemic: an exploratory analysis of eIR and eLMIS in Guinea, Honduras, India, Rwanda and Tanzania
This report presents a series of brief case studies supported by the existing literature on the deployment of digital solutions during the COVID-19 pandemic. It specifically describes how electronic Immunization Registries (eIR) and electronic Logistics Management Information Systems (eLMIS) have been newly developed and/or repurposed in Guinea, Honduras, India, Rwanda, and Tanzania. Importantly, the findings are influenced by the state of the implementation which, because of the delayed availability of COVID-19 vaccines in the LMICs, is still in its early phase.
Annual Evaluation Reports
Annual Evaluation Report 2024
Gavi, the Vaccine Alliance produced its annual evaluation report for 2024, which offers an overview of both recently completed and ongoing centralised and decentralised evaluations, along with updates on efforts to strengthen Gavi’s evaluation function.
Annual Evaluation Report 2023
Gavi, the Vaccine Alliance produced its annual evaluation report for 2023, which offers an overview of both recently completed and ongoing centralised and decentralised evaluations, along with updates on efforts to strengthen Gavi’s evaluation function.
Annual Evaluation Report 2022
Gavi, the Vaccine Alliance produced its annual evaluation report for 2022, which offers an overview of both recently completed and ongoing centralised and decentralised evaluations, along with updates on efforts to strengthen Gavi’s evaluation function.
Gender and digital health information in immunisation programming
This Technical Brief provides a summary of the state of evidence and priority areas for investment and attention at the intersection of gender, immunisation and digital health and data that have been used to inform the development of Gavi’s Digital Health Information Strategy, which includes gender intentional strategies, outcomes, outputs, and inputs for global and country consideration and action.
Sub-national multi-source data for immunisation programme decision-making
This Technical Brief provides a review of the state of evidence and experiences with sub-national data use for decision-making, identifies gaps and makes recommendations to inform the development of Gavi’s Digital Health Information Strategy. Drawing from literature, evidence, key informant interviews and Gavi’s DHI prioritisation exercise, the document provide recommendations as well.
COVID-19 innovations and digital applications for routine immunisation
This Technical Brief provides a summary and review of experiences with new and adapted digital solutions for COVID-19 vaccine delivery at the global, regional and country level to ensure that the lessons, challenges and successful application of digital health information innovations are captured as part of Gavi’s Digital Health Information Strategy.
Last updated: 13 Feb 2026