Contents
VIS analyses: capturing the full value of vaccines
VIS role in assessing threat of outbreaks, epidemics and pandemics
VIS 2024: governance documents
Update on previous VIS (VIS 2018)
Share
Contact
For further information about the VIS process, please contact VIS@gavi.org.
Introduction to Gavi’s VIS
Beginning with the first successful vaccine against smallpox in 1796, to the latest vaccines against pathogens such as COVID-19 and malaria, new vaccines are continually being developed, tested and rolled out to save lives. Every five years, Gavi takes stock of the vaccine landscape to assess the impact, cost, value and programmatic feasibility of underused or new vaccines of highest relevance to Gavi-eligible countries via the Vaccine Investment Strategy (VIS). The VIS sets new priorities for Gavi’s vaccine support programmes in a transparent manner through in-depth, evidence-based analysis and extensive consultations, including with independent experts.
Impact of VIS
Led by Gavi’s Policy team, the VIS process has helped increase Gavi’s portfolio from vaccines against 6 infectious diseases during its 2001–2005 strategic period to 19 today – including Board approvals for Ebola vaccine in 2019, COVID-19 vaccine in 2021 and malaria vaccine in 2022.
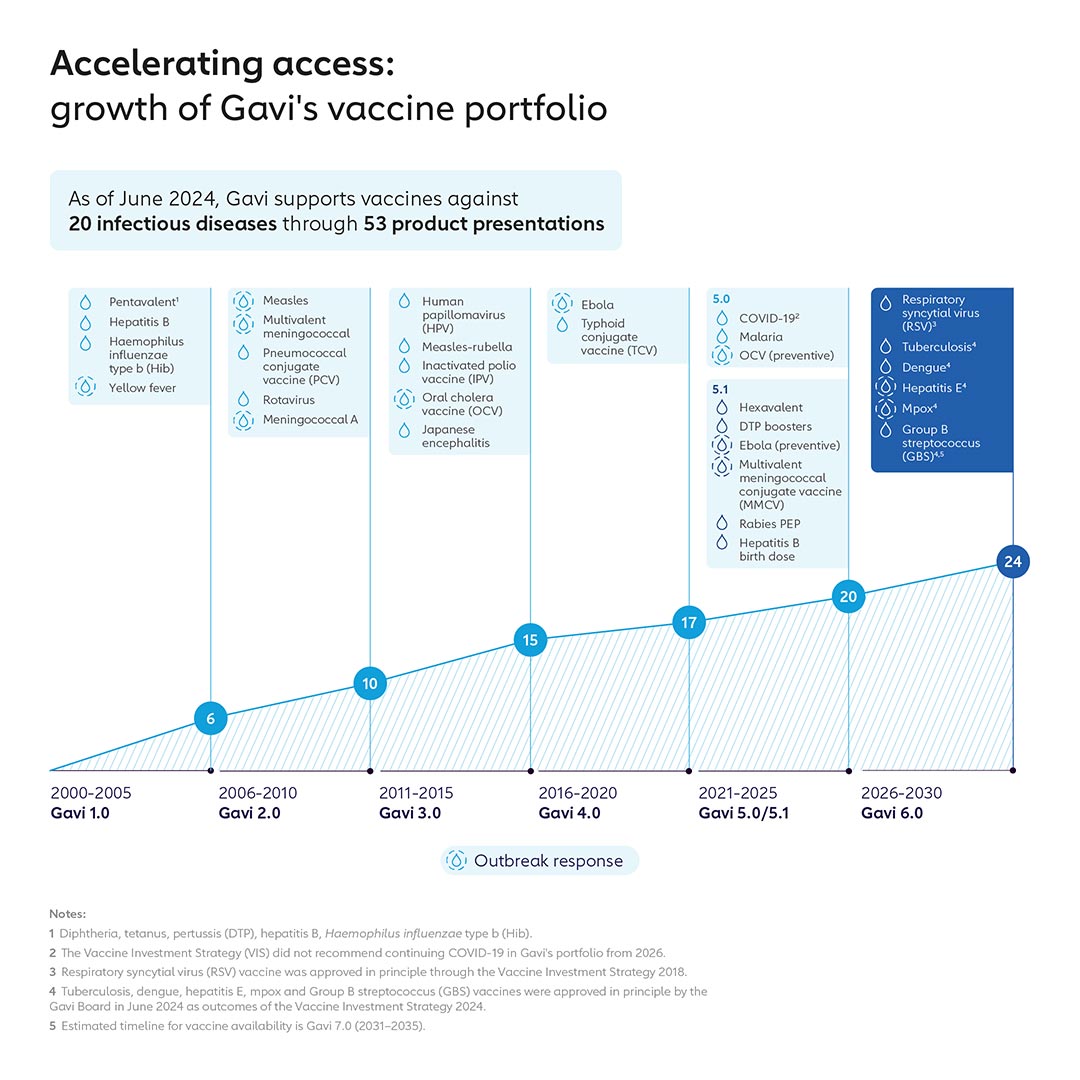
Gavi’s disease prevention focus and vaccine portfolio have evolved over time, moving from vaccines focused on routine infant immunisation to prevent endemic disease to include vaccines that target other age groups, such as HPV, as well as vaccines that support outbreak, epidemic and pandemic preparedness and response (PPR) through the establishment of global stockpiles.
FIVE-YEAR CYCLES
The VIS operates on a five-year cycle to provide Gavi implementing countries, manufacturers, donors and other partners with predictability and information to support long-term strategic planning of immunisation programmes, as well as our upcoming strategic period. The process is focused on the needs and priorities of lower-income countries. The five-year time span reflects the long lead time required to develop; demonstrate technical success and model impact of; and approve vaccines.
VIS: informing Gavi’s future investments
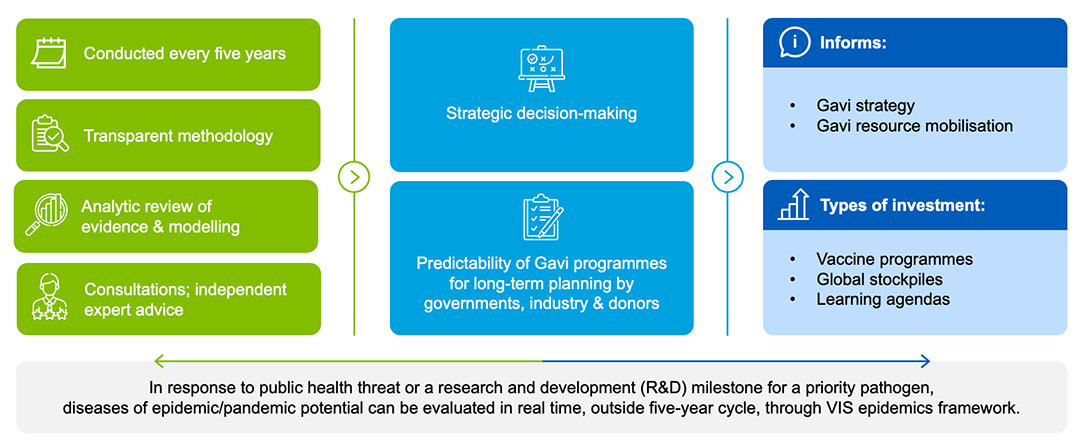
VIS 2024 timelines and scope
Each VIS process has been tailored to reflect the current global context and Alliance strategic priorities, as well as the availability of data and improved methods for assessing impact. VIS 2024 will be no exception – aligning with Gavi’s strategic priorities and considering innovative vaccine technology; a post-pandemic environment; impact of climate change; human-animal interface; as well as demographic transition, urbanisation and rise of antimicrobial resistance (AMR).
In late 2022, Gavi initiated the planning for VIS 2024; in June 2024, the Gavi Board will take a final decision to inform Gavi investments between 2026–2030. The VIS process is overseen by Gavi’s governance structures: the Programme and Policy Committee (PPC) reviews, monitors and makes recommendations to the Gavi Board, which then makes the final decisions on VIS investments.
The assessment and evaluation of vaccine candidates is completed in three phases and Board decisions:
Phase 1:
- generate longlist of potential investments, working with the World Health Organization (WHO) and based on pre-established inclusion criteria (see below)
- develop evaluation frameworks against which to evaluate longlist products based on feedback from the VIS Steering Committee and other external consultations
- demand forecasting and impact modelling
First Board decision: approval of longlist and evaluation frameworks.
Phase 2:
- analyse longlist products according to evaluation framework to narrow to shortlist – again with insight from the VIS Steering Committee and other external consultations
Second Board decision: approval of shortlist of vaccines to be developed into investment cases.
Phase 3:
- develop detailed investment cases (including modelling of potential costs and impact; proposed learning agendas or outstanding questions that would inform the roll-out of a successful vaccine programme; health system support; diagnostic/test support; as well as input from disease experts and other external consultations) for the shortlist vaccines, comparing against each other and existing portfolio vaccines to provide basis for final Board decisions
- consider during investment case development Gavi’s comparative advantage and fit with anticipated strategic priorities.
Third Board decision: final decision on investment cases
VIS 2024 timelines and process
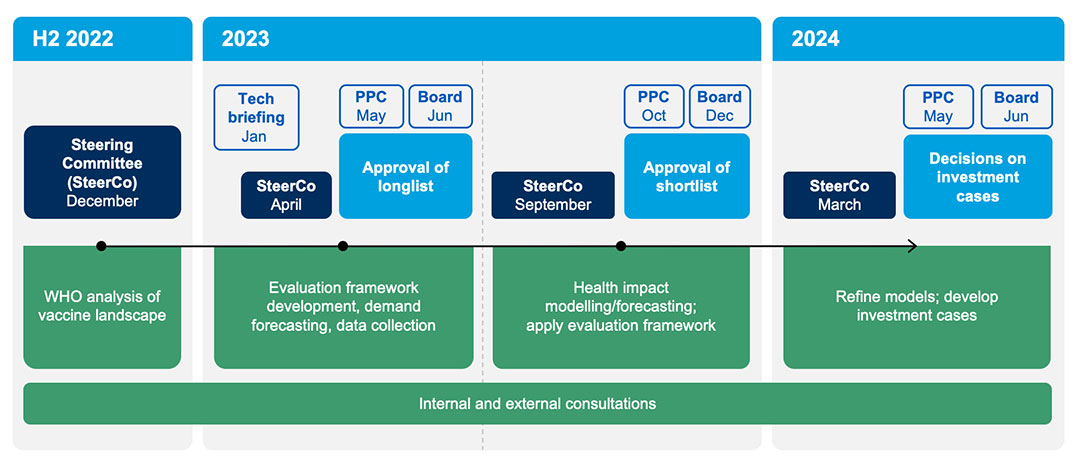
The VIS process begins with an analysis of the vaccine landscape carried out by the World Health Organization (WHO) using the following inclusion criteria:
- immunisation products of relevance to Gavi-eligible countries with expected licensure by 2030
- vaccines of relevance to Gavi-eligible countries not currently in Gavi’s portfolio, or incremental investments for Gavi-supported vaccines
VIS 2024 is in close alignment with the development of the Gavi 6.0 strategy to ensure that the outcomes of VIS 2024 respond to and inform the strategic priorities defined in Gavi 6.0, which is scheduled for Board review in June 2024.
VIS analyses: capturing the full value of vaccines
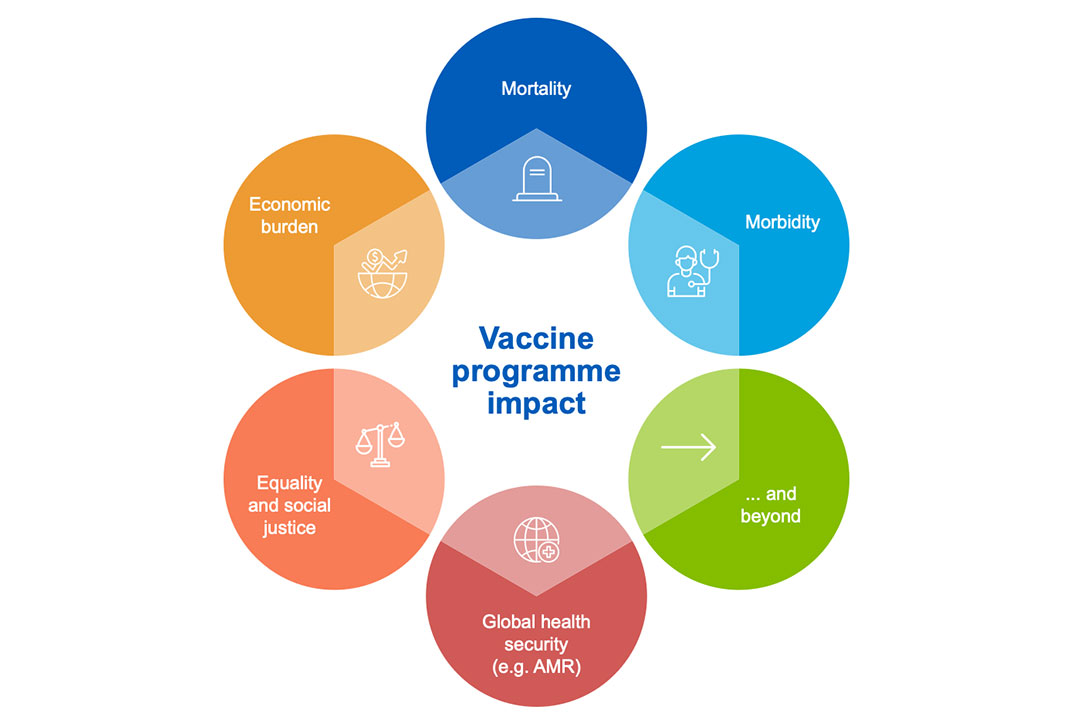
VIS analyses are based on evidence and data that together provide an understanding of the full value of vaccines, beyond their impact on mortality and morbidity – to include social, economic and population health benefits. Our analyses help: inform the evaluation of pipeline vaccines and vaccines not currently in Gavi’s portfolio; determine the most relevant investment type; and understand potential designs for learning agendas and further data collection.
VIS analyses review the burden of disease and identify the most appropriate vaccination strategy for Gavi-eligible countries to enable demand forecasting. These data inputs are used to model health impact and costs, while implementation feasibility is also considered. Finally, vaccine candidates are compared to one another across these different factors, and to the current portfolio, to arrive at investment decisions. The VIS is highly consultative and inclusive, drawing on the breadth of multidisciplinary expertise of Alliance partners and other stakeholders – including countries, donors, civil society organisations (CSOs), disease experts and modelers – to reflect diverse perspectives.
18-month VIS process: robust, evidence-based
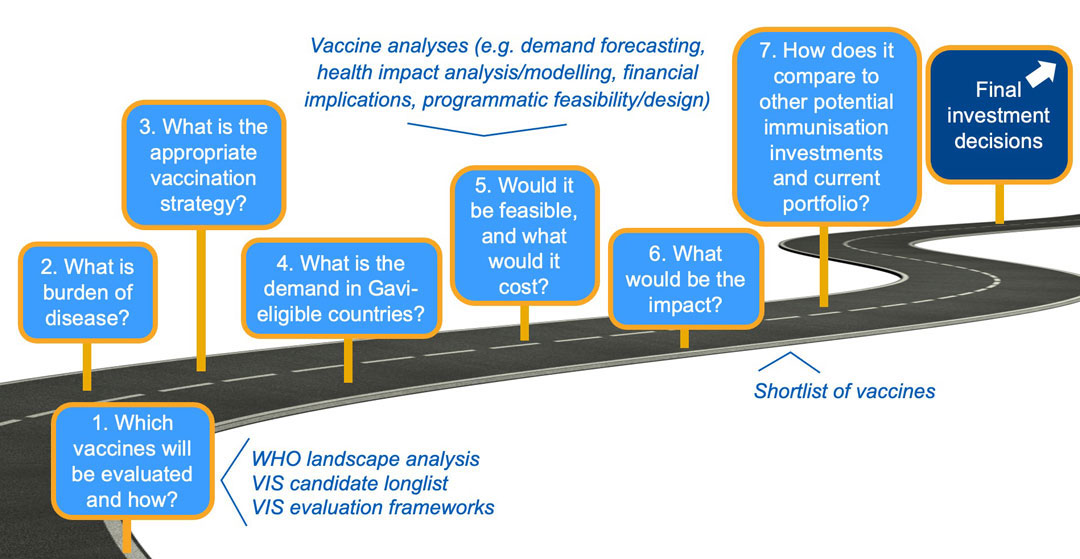
These analyses are structured around an evaluation framework that is developed for each VIS. The framework reflects Gavi’s core mission (i.e. save lives and protect people’s health), Alliance stakeholder strategic priorities, as well as a review of the previous evaluation approach for continued fit and relevance. In addition, analyses are conducted on specific topics to identify opportunities and perspectives on additional measures of value and impact. The framework includes criteria and indicators that can be quantitative or qualitative and that are weighted differently to inform the prioritisation process.
The data we use to analyse vaccine products is highlighted below. VIS data requirements align closely with those outlined in the WHO Full Value of Vaccines Assessments (FVVA), as well as the Evidence Considerations for Vaccine Policy Development (ECVP).
VIS evaluation frameworks: broad understanding of return on investment
The VIS has two evaluation frameworks: one for endemic diseases and one for epidemic diseases. The public health goal of Gavi’s potential vaccine investment informs the evaluation framework that is used.
VIS role in assessing threat of outbreaks, epidemics and pandemics
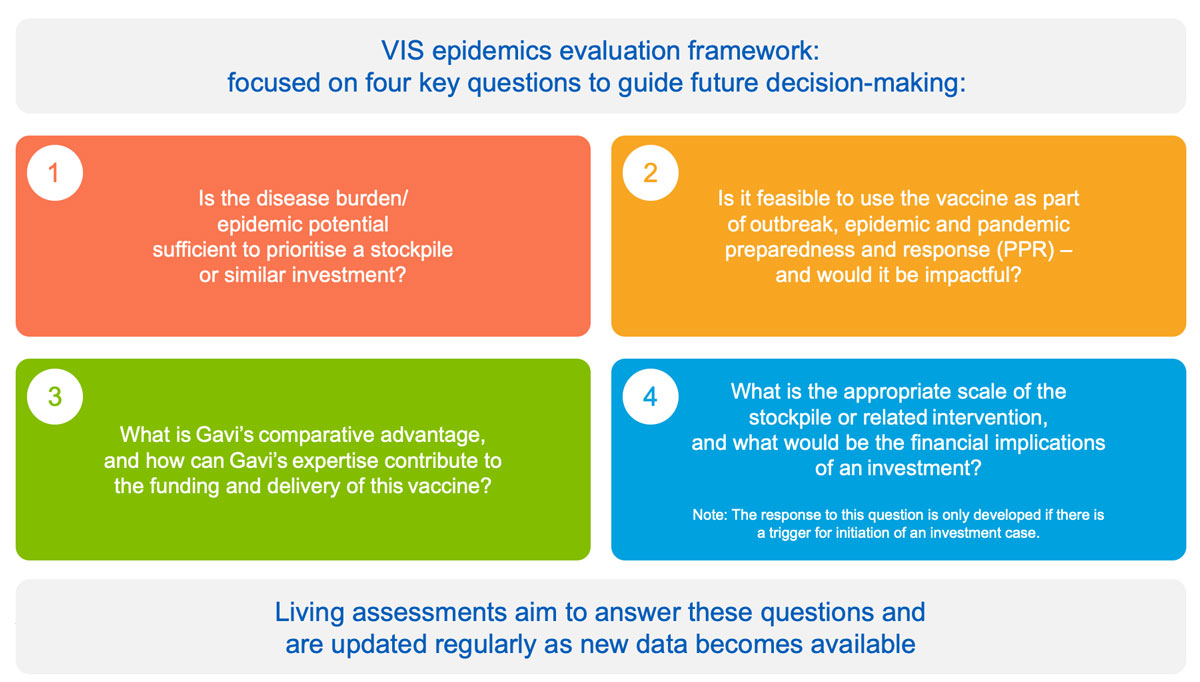
While the VIS process includes products for outbreak, epidemic and pandemic preparedness and response (PPR), these can also be evaluated in real-time when needed to facilitate agile decision-making in context of a public health event or R&D milestone. The epidemics process in based on:
- an epidemics evaluation framework structured around four key questions to inform future investment cases: disease risk and burden; role of vaccine; Gavi comparative advantage; and overall financial cost
- development of “living assessments” for priority pathogens identified by WHO R&D Blueprint and CEPI – a tool to monitor emerging data; highlight data gaps; and facilitate timely decision-making upon epidemic and/or market triggers. Living assessments are developed for relevant vaccines until an investment case is triggered.
VIS 2024 Steering Committee
The VIS Steering Committee is convened prior to each PPC meeting (i.e. December 2022, March 2023, September 2023, March 2024) to deliver independent expert advice to the Gavi Secretariat across multiple perspectives. As per the Terms of Reference, the VIS Steering Committee will:
- provide guidance on the strategic questions, methodology and process for the VIS;
- provide guidance on the evaluation framework, criteria and weightings;
- validate assumptions and outputs of analyses and models for each disease/vaccine; and
- support strategic thinking on emerging recommendations prior to Gavi Board and PPC decision points.
The VIS Steering Committee is comprised of 20 individuals, including a Chair with strong technical and/or scientific expertise and broad-ranging perspectives on aspects pertinent to VIS analyses and decisions. Type of expertise includes, but is not limited to, epidemiology, health impact analysis/modelling, economic evaluations, vaccine implementation and service delivery, research and development, and health financing and planning. Members have been either: selected based on their individual expertise from a pool of applicants to an open call for expressions of interest; or appointed by the Secretariat ex officio to represent partner institutions and stakeholders – including two members of Gavi’s PPC (to serve as liaisons, providing strategic guidance and ensuring alignment between the committees); and a civil society organisation (CSO) representative.
LIST OF VIS 2024 STEERING COMMITTEE MEMBERS
Independent experts
| Helen Rees, Chair Wits RHI |
| Alejandro Cravioto Universidad Nacional Autónoma de México |
| Azra Ghani Imperial College London |
| Mark Jit London School of Hygiene & Tropical Medicine |
| Ruth Karron Johns Hopkins Bloomberg School of Public Health |
| Sonali Kochhar University of Washington, Seattle |
| Tawanda Marufu University of Zimbabwe College of Health Sciences |
| Amani Mustafa The Carter Center Sudan |
| Rajinder Suri Developing Countries Vaccine Manufacturers Network |
| George Warimwe KEMRI - Wellcome Trust Research Programme |
| Meseret Zelalem Ministry of Health Ethiopia |
Observers

VIS 2024: decisions
In June 2024, the Gavi Board approved VIS 2024, which will include future vaccine programmes and learning agendas for tuberculosis (adolescent vaccines), Group B streptococcus (GBS), and dengue (the latter investment conditional on availability of relevant data on the burden of disease in Gavi-supported countries), as well as global stockpiles and learning agendas for vaccines against mpox and hepatitis E.
In-principle approvals via the VIS allow Gavi to begin market signaling and preparedness activities in advance, which is a critical part of Gavi's model to accelerate access to lower-income countries so new vaccines can be deployed as soon as they become available. Learning agendas are research implementation questions that Gavi can support to provide evidence for the launch, implementation and effective scale-up of a vaccine programme or stockpile.
The Gavi Board also approved a learning agenda on vaccination against Shigella and, given the levels of coverage achieved, current epidemiology and country demand, recommended sunsetting the routine COVID-19 vaccine programme at the end of 2025. Potential COVID-19 vaccine needs for a ‘worst-case scenario; after 2025 will be addressed through Gavi's emergency response mechanisms. Previously, the Board had not shortlisted chikungunya vaccines for further consideration; however, should the need arise, an investment in chikungunya vaccines could be re-evaluated through Gavi’s living assessment process.
For mpox, the Board additionally approved that in the current strategic period, the Vaccine Alliance would support the response to the ongoing outbreaks in the Democratic Republic of the Congo (DRC) and surrounding countries – including leveraging COVID-19 experiences to coordinate dose donations.
Investment cases for these vaccines can be found below.
Summary of Board decisions for VIS 2024
| Vaccine | Population | In-principle investment | Learning agenda |
|---|---|---|---|
| Tuberculosis | Adolescents and adults | ✓ | ✓ |
| Group B streptococcus | Pregnant women | ✓ | ✓ |
| Dengue | 2–16-year-olds Conditional on burden data in Africa | ✓ | ✓ |
| Hepatitis E | High-risk populations in outbreak response | ✓ | ✓ from Gavi 5.1 |
| Mpox | ✓ | ✓ from Gavi 5.1 | |
| Shigella | Infants | ✗ | ✓ |
| Chikungunya | Outbreak response | ✗ | ✗ |
| COVID-19 | High-risk populations | No continued investment post-2025 | |
Link to investment cases
VIS 2024: governance documents
- VIS 2024 Board Paper 1: Longlist and evaluation frameworks
- VIS 2024 Board Paper 2: Proposed shortlist
- VIS 2024 Board Paper 3: Investment cases
- VIS 2024 Annex to Board Paper 3: Summary of recommendations and costs
Update on previous VIS (VIS 2018)
In December 2018, the Gavi Board approved six new vaccine programmes for inclusion in the 2021–2025 strategic period: rabies; hepatitis B birth dose; diphtheria, tetanus and pertussis (DTP)-containing boosters; respiratory syncytial virus (RSV); preventive cholera; and multivalent (A, C and W, Y & X-containing) meningococcal conjugate vaccines (MMCV).
In December 2020, due to the COVID-19 pandemic, the Gavi Board paused or delayed availability of funding for vaccines that had been approved for inclusion in the 2021–2025 strategic period as part of the previous VIS (VIS 2018): rabies; hepatitis B birth dose; and DTP-containing boosters to focus on maintaining routine immunisation; reaching zero-dose children and missed communities; and establishing mechanisms to support future access to and delivery of COVID-19 vaccines. No relevant RSV product was available for introduction at the time.
The pause was not applied to preventive cholera or multivalent meningococcal conjugate vaccines, considering their outbreak response potential and the fact that they built on existing Gavi-supported vaccine programmes.
In 2023, the PPC provided guidance for a sequenced unpausing of vaccines. A vaccine programme for DTP-containing boosters was launched in December 2023; and for rabies and hepatitis B birth dose in June 2024. MMCV became available in July 2023; and the funding window opened in June 2024.
Investment cases for VIS 2018 vaccines:
- Diphtheria Tetanus and Pertussis-Containing Boosters Investment Case
- Hepatitis B Birth Dose Investment Case
- Multivalent Meningococcal Investment Case
- Oral Cholera Investment Case
- Rabies Investment Case
- Respiratory Syncytial Virus Investment Case
- Presentation to the Gavi Board, June 2018
- Report to the Gavi Board, November 2018