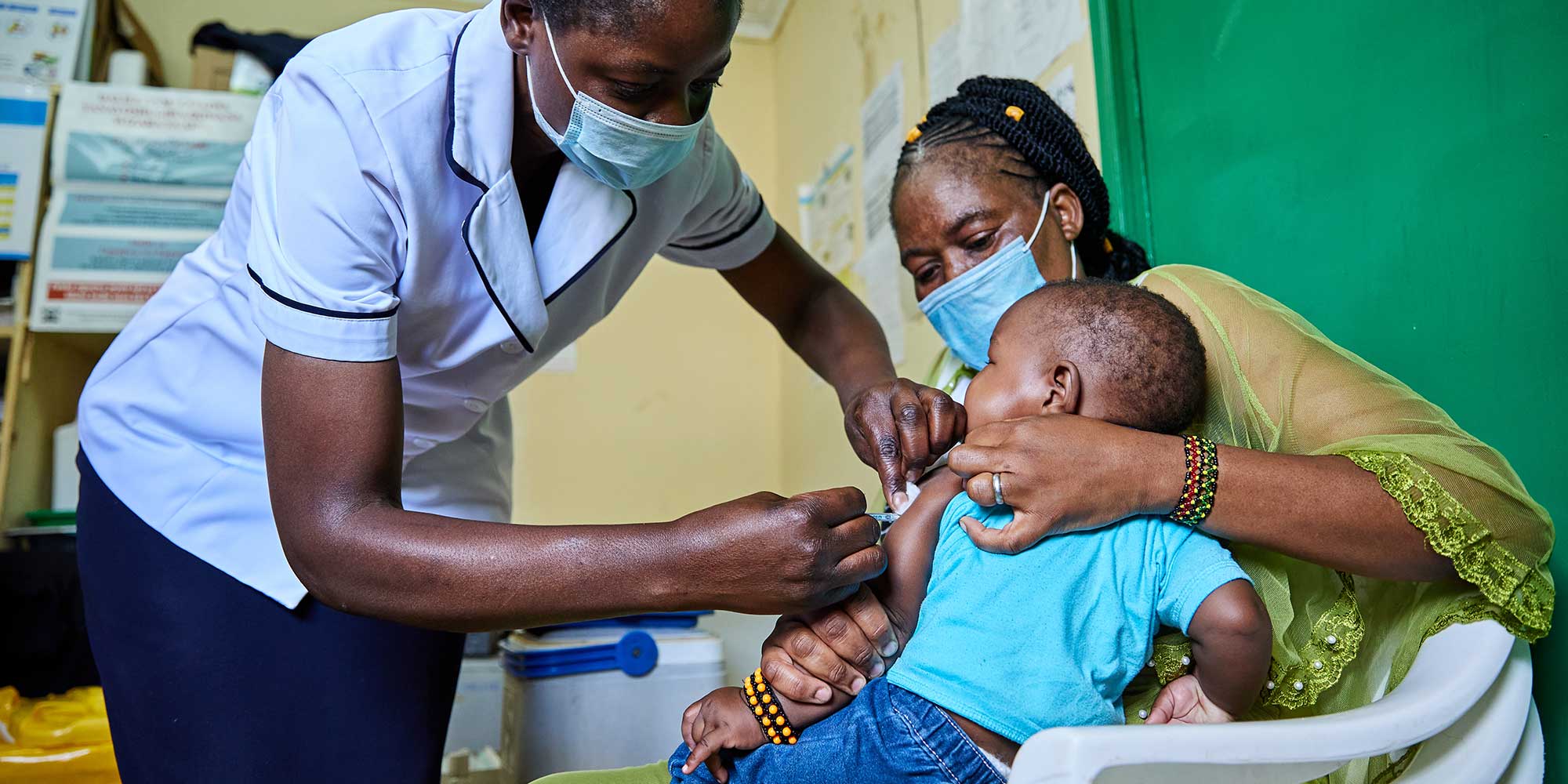White Paper
April 2023
Contents
The long road to the world’s first effective malaria vaccine
Shaping the market to increase supply
Facilitating supplier diversification
Understanding demand to enable vaccines to fulfil their potential
Maximising the impacts of vaccines through innovation
Disclaimer:
This document is published by Gavi, the Vaccine Alliance. The contents included is the result of a collaborative process managed by Gavi. The republication or usage of the content of this document of any kind without written permission is prohibited. Please contact media@gavi.org with any requests for use.
Share
Executive summary
An estimated 475,000 children under the age of five died of malaria in the World Health Organization (WHO) African region in 2021. That’s around three-quarters of the worldwide total of lives lost to the disease. Since 2000, the global death toll has fallen by almost a third thanks to the use of mosquito nets, insecticides and drugs. However, in 2015 this progress began to stall and deaths have since increased, in 2020 and 2021 by more than 10% compared to 2019.
Given the huge potential benefits, scientists have been trying to develop a malaria vaccine for more than 50 years. However, doing so has proved especially challenging because the Plasmodium parasites that spread the disease take on different forms during their lifecycle, complicating the task of finding vaccine targets. RTS,S/AS01e, developed and manufactured by GlaxoSmithKline (GSK) and which took more than three decades to develop, is the first vaccine to be shown in trials to be safe and effective against malaria.1 In October 2021, the WHO recommended the use of RTS,S/AS01e in children in regions with moderate to high Plasmodium falciparum malaria transmission, the majority of which occurs in Sub-Saharan Africa. This was a truly historic breakthrough. A second malaria vaccine, R21/Matrix-M, developed by Oxford University and to be manufactured by Serum Institute of India (SII), is also in ongoing phase 3 trials, with WHO recommendation and prequalification hoped for in the near term.
Trials have shown the RTS,S/AS01e vaccine can reduce cases of severe malaria in children aged 5-17 months by 30%. Researchers estimate that RTS,S/AS01e could prevent the deaths of 24,000 children a year if global vaccine supplies were to reach 30 million doses. It is hoped that other malaria vaccines, currently in ongoing trials or at earlier phase of development, will further increase the numbers of lives that can be saved. Publication of the initial phase 3 trial results for the R21/Matrix-M vaccine is expected imminently.
Today, malaria is a tropical disease mainly affecting lower-income countries, many of which are eligible for Gavi support. Given the significant anticipated demand for malaria vaccines, and recognition that there will be limited supplies in the early years of the Gavi programme, securing a healthy market for malaria vaccines is of critical importance. One of the primary reasons Gavi was created was to address the failure of markets to provide the incentives for manufacturers to invest in producing new vaccines at affordable and sustainable prices for low- and middle- income countries.
The Gavi Board decision in 2021 to establish a malaria vaccine programme, with introductions primarily expected to take place in sub-Saharan African countries where the greatest burden of Plasmodium falciparum malaria occurs, has helped establish a nascent market for these vaccines. On top of this, for several years Gavi had already been implementing market shaping efforts to improve the supply outlook. This includes the 2021 innovative financing agreement between Gavi, GSK, and MedAccess, which guaranteed the continued production of the RTS,S antigen component pending the WHO recommendation and the Gavi Board decision, thereby averting any further delays in eventual supply availability. In addition, Gavi, along with Unitaid and the Global Fund, has been providing funding support to the WHO-coordinated Malaria Vaccine Implementation Programme (MVIP), generating key evidence needed to inform global recommendations on the use of RTS,S/ AS01e, inform the design of what at the time was a potential malaria vaccine programme and establish demand for malaria vaccines. In January 2023, Gavi published the Alliance’s Market Shaping Roadmap, setting out the Alliance’s plans to work together and with other stakeholders to improve the health of the malaria vaccine market based on the following objectives and targets:
- Increase vaccine supply so that it meets demand as soon as possible, and no later than 2026.
- Increase the number of vaccine suppliers, including at least one that manufactures in Africa in the medium- to long-term (3-15 years), and dramatically reduce the price of malaria vaccines.
- Improve understanding of vaccine demand, help implementing countries ensure they are ready for vaccine introduction by the time supplies are available and ensure countries continue their malaria vaccination programmes after they transition out of Gavi support.
- Establish an enabling environment for innovation in both existing and pipeline products.
The long road to the world’s first effective malaria vaccine
Malaria is caused by Plasmodium parasites, which are spread to people through the bites of infected female Anopheles mosquitoes. There were an estimated 247 million cases of the disease and 619,000 malaria deaths worldwide in 2021. More than 76% of the lives lost are in children under the age of five in the WHO African region.
The global malaria death toll has fallen over the last two decades, from almost 900,000 annually in the year 2000, thanks to the use of insecticide-treated mosquito nets and indoor residual spraying, as well as the use of preventative chemotherapies in vulnerable populations and antimalarial medicines. More recently, however, this progress has stalled, with the estimated number of cases having dropped by only 3% between 2015 and 2020 and then rising in both 2020 and 2021, according to the WHO.
Alongside other recommended interventions, malaria vaccines have the potential to reduce morbidity and mortality significantly. Scientists have been working on them for several decades, however the complicated, multi-stage lifecycle of Plasmodium parasites and their ability to evade immune system detection have made doing so especially challenging. In October 2021, WHO recommended for use the first vaccine for the prevention of P. falciparum malaria with prequalification of the vaccine announced in July 2022.
Called RTS,S/AS01e, the vaccine targets the sporozoite form of the parasites that are injected into the blood of humans when they are bitten by infected mosquitoes. This approach was developed in a collaboration between the Walter Reed Army Institute of Research (WRAIR) in the US, and SmithKline and French, a predecessor to GSK, during the 1980s. GSK’s adjuvant AS01e is used to boost immune responses to RTS,S, while R21/MatrixM uses Novavax’s adjuvant Matrix-M which has a similar mechanism of action to induce an immune response against Plasmodium falciparum malaria and boost immunity.
The ongoing Malaria Vaccine Implementation Programme (MVIP), which was launched in Ghana, Kenya and Malawi in 2019, showed that after 24 months of immunisation with four doses of RTS,S/AS01e there was a substantial reduction in severe malaria – a reduction in child hospitalisations and a drop in child deaths. During 2022, Gavi approved funding to support continued implementation of vaccination programmes in the three countries that took part in the MVIP once the MVIP concludes at the end of 2023. Non-pilot countries with moderate to high transmission of P. falciparum malaria were able to first apply in January 2023, and another application for countries seeking such support was opened in April and a third is due in July 2023. In December 2021, Gavi announced plans to invest in malaria vaccine introduction, procurement and delivery. RTS,S is the first vaccine to be included as part of Gavi’s malaria vaccine programme, and additional malaria vaccines will be added to Gavi’s programme as soon as they are prequalified and recommended for use by the WHO.
Box 1: What is Market Shaping?
The scientific challenges involved are not the only reasons why it has taken several decades to develop and deploy an effective malaria vaccine. As with other vaccines intended primarily for use in low- and middle-income countries, manufacturers have faced uncertain returns on investment, which is one of the reasons why vaccines have historically either not made it to market at all, or only become available in low- and middle-income countries long after they have been introduced in wealthier countries, and illustrates how market forces can fail to serve the interests of low-income countries.
Gavi was created to expand access to childhood immunisation and one key way in which it does this is by addressing vaccine market failures. This deliberate approach to shaping multiple vaccine markets over more than 22 years, together with a long horizon perspective, has enabled Gavi to help create sustainable and healthy markets, giving it unparalleled experience in the field. Gavi’s strategies aim to foster: a sustainable and competitive supplier base; healthy demand driven by a supportive environment; and an environment that encourages transformational innovation. Gavi has a successful track record of devising and implementing interventions to achieve these goals in ways that improve the supply of vaccines for infectious diseases at affordable, sustainable prices for developing countries. These include: incentivising innovative vaccine product development; working with implementing countries to improve the accuracy of demand forecasting; designing innovative procurement mechanisms (including those that leverage the production economics of high demand volumes to bring down prices); bilateral and multi-party risk-sharing agreements; discretion to accommodate multiple price points in key markets; and close working relationships with manufacturers.
Shaping the market to increase supply
The supply of malaria vaccine will be constrained initially. More than 30 countries are host to areas with moderate to high P. falciparum malaria transmission where the vaccine has the potential to provide protection to more than 25 million children born each year. Given the impact of the disease in areas of high burden, not just in terms of mortality but also the socio-economic impact – with children often experiencing several episodes of the disease, and with recurring sickness taking many adults out of work for prolonged periods – dozens of countries have expressed an interest in introducing the vaccine. However, without the interventions that are being taken by Gavi and Alliance partners, it may take a number of years before supply can match this demand. Therefore, until vaccine supply is increased once a second vaccine become available and as RTS,S production is increased, to begin with supplies will be carefully targeted at populations in areas of greatest need.
Initial supplies of RTS,S/AS01 are expected to be distributed based on the principles set out in the Framework for the allocation of limited malaria vaccine supply. This outlines how priority will be given to areas where: the malaria burden and risk of death in children is highest; the most lives can be saved based on factors including the ability to ensure children receive all recommended doses; there is a commitment to fairness and addressing the needs of marginalised individuals and communities; individuals or groups have contributed to the clinical development of this vaccine.
The Alliance’s Market Shaping Roadmap for malaria vaccines, published in January 2023, sets out the Alliance’s main objectives and outlines a number of ways these can be achieved. Its top priority is working, alongside its partners, to increase the supply of vaccine so that this meets demand as soon as possible, and no later than 2026, and to significantly reduce the price of malaria vaccines.
Gavi will seek to increase vaccine supply by:
- Contracting activities to lock in as much supply as possible, and follow-up to ensure contracted volumes of vaccine are delivered to countries.
- Ongoing engagement with suppliers to help them scale up production by providing updated forecasts and market and programme updates.
- Supporting efforts to accelerate regulatory approvals and WHO prequalification and the provision of new or updated policy recommendations.
- Helping manufacturers working on new vaccines to speed their development and approval.
In partnership with GSK and MedAccess, Gavi established an innovative financing agreement to ensure continued production of the RTS,S antigen in 2021. Following a tendering process, UNICEF awarded its first contract to supply malaria vaccines in 2022. Under the agreement, GSK expects to deliver 18 million doses, made up of 4 million doses by late 2023, 6 million doses in 2024 and 8 million in 2025. UNICEF expects to award contracts for additional supply as new vaccines receive licensure and are prequalified and recommended by the WHO.
Beyond that, production of RTS,S/ASO1e is expected to increase to 15 million doses per year during 2026-2028 thanks to a product transfer deal with Bharat Biotech, of Hyderabad, India. Initially this will take over secondary activities such as freeze-drying, filling vaccine vials and packaging using RTS,S antigen and AS01e adjuvant provided by GSK. Under the agreement, Bharat aims to take over manufacturing the antigen and production of the vaccine under licence with adjuvant supplied by GSK no later than 2028.
However, RTS,S vaccine production will still fall short of demand. Other malaria vaccines are needed to fill the gap. One of Gavi’s key objectives, as outlined in its Market Shaping Roadmap, is for there to be at least two malaria vaccines that have been licensed and prequalified by no later than 2025. The most advanced candidate is R21/Matrix-M, being developed by a team at Oxford University, in the UK, and manufactured by the Serum Institute of India. This is currently undergoing phase III trials in Burkina Faso, Kenya, Mali and Tanzania, and results relating to safety, efficacy, and duration of protection in different malaria transmission settings are imminently pending. A WHO working group of global immunisation and malaria advisers has started reviewing available R21 vaccine safety and efficacy data from the ongoing phase 3 trial of the vaccine to consider a potential policy recommendation. The vaccine is also being reviewed for WHO prequalification. If recommended and prequalified, this would enable it to be available in areas with moderate to high P. falciparum malaria transmission, potentially with an initial limited indication depending on the scope of any WHO recommendation, as early as 2024.
Both the arrival of a second vaccine R21/MatrixM, and the product transfer of RTS,S, along with their production in India, have the potential to significantly increase the supply of doses, and bring down prices. So too does the development of other pipeline candidate vaccines. There are dozens of studies under way focused on malaria vaccine development, although as of 2021 around 90% of these were at the discovery, pre-clinical or phase I trial stage. German biotech company BioNTech, for example, is seeking to apply the mRNA technology it used to develop its COVID-19 vaccine to a malaria vaccine. It announced the launch of a phase I trial of its candidate, called BNT165b1 in December 2022.
Facilitating supplier diversification
A competitive and sustainable supplier base is key to healthy vaccine markets. It increases production volumes, drives down prices, protects against market shocks and individual supplier risks, and, ultimately, makes it possible to save more lives. That’s why the diversification of supply in the medium- to long-term (2025-2036) is another key objective of Gavi’s Market Shaping Roadmap, with an aim of there being multiple suppliers including at least one producing a malaria vaccine in Africa.
Another of Gavi’s market-shaping goals is a significant reduction of prices in the medium-term (e.g., by 2028). The current price for the malaria vaccine, as with many other vaccines when first launched, is relatively high, in part because production is still being scaled up. Economies of scale will help lead to lower prices over time, as well as the shifting of production of malaria vaccines to India and/or other LMICs. Previous experience shows that implementing countries can expect the scale-up of production and entry of additional manufacturers into the market to drive prices down, as happened in the case of the pneumococcal conjugate vaccine (PCV) following the launch of an Advance Market Commitment by Gavi, WHO, UNICEF and the World Bank in 2009. Beyond that it is hoped that the introduction of new and improved products will result in further price decreases. The initial high price of the first malaria vaccine is not intended to set a precedent for any other vaccine to be priced similarly.
The initial high price and recommended four-dose schedule for RTS,S/AS01e means the cost of the vaccine could present obstacles to its successful introduction in some countries, and similarly these types of challenges may impact other malaria vaccine candidates as well. To address this, the Gavi board adopted in December 2022 a new policy that included a special approach to co-financing of malaria vaccines.2 This means that countries in Gavi’s initial self-financing phase under Gavi’s eligibility and transition policy will pay just US$ 0.20 per dose, while those in the preparatory transition phase will pay US$ 0.20 per dose to begin with, followed by annual increases of 15%. Countries in Gavi’s accelerated transition phase (ATP) will start to pay 20% of the price (irrespective of what year they are in their accelerated transition phase).3 Countries in the ATP phase will have eight years to scale up payments until the ninth year when they will cover 100% of the vaccine costs, which is longer than under previous Gavi vaccine support programmes. Providing generous financial support to countries seeking to introduce malaria vaccines can prevent cost becoming an obstacle to access, and, in turn, incentivise supply diversification and vaccine innovation.
Understanding demand to enable vaccines to fulfil their potential
Supply is, of course, only one half of the equation. Predicting levels and timing of vaccine demand and matching these to supply can be challenging. If manufacturers lack confidence in demand forecasts, and if this is based on previous experience, there could be a risk this could undermine their incentives to increase supply.
Gavi harnesses the specialist knowledge of partners and other experts to produce vaccine-specific strategic demand scenarios (SDS) to improve understanding of vaccine markets. These are primarily aimed at facilitating important medium- and longer-term decisions, including those made by key stakeholders such as manufacturers and implementing countries. They can inform supply and procurement strategies, as well as highlight challenges in specific markets. Demand for malaria vaccines is predicted to be around 80-100 million doses per year by 2030, based on the 2021 Gavi SDS forecasts.
Implementing countries also have a key role to play in helping improve the health of the malaria vaccine market, particularly in establishing timely and predictable demand for malaria vaccines. Part of that will involve ensuring that funding application submissions and vaccine introductions take place on the original timelines indicated by countries and that are used to create the Alliance’s vaccine market forecasts, as this is critical to reinforce the message to malaria vaccine suppliers to scale up supply availability quickly. Countries can also help by submitting quality and timely applications for funding for review and approval by the Gavi Independent Review Committee (IRC). Countries are already doing this. A total of 14 countries have already been recommended by the Gavi IRC for approval of their malaria vaccine introduction applications as of the end of March 2023. Healthy vaccine markets also require that countries implement necessary preparations and activities to ensure immunisation infrastructures and systems are ready to deliver the vaccine quickly to those who need it as and when malaria vaccine supply becomes available.
Low or delayed application submissions or delayed introductions may signal to the market that additional supply needs are not as high as forecasts indicate, which may lead to lower future available supply than is needed. It can also reduce the impact of immunisation programmes and act as an obstacle to equitable vaccine coverage, while initiatives designed to encourage people to take up vaccination can increase demand. These need to address context-specific challenges and socio-behavioural considerations, including the challenges involved in introducing a four-dose vaccine that falls outside traditional routine immunisation schedules. Similarly, health workers implementing malaria vaccine programmes may need additional support in improving communication skills and quality of care. Robust monitoring and evaluation to inform learnings and improvements to deliver the vaccine is also required.
Alliance partners will also play their part in helping implementing countries ensure that real demand matches forecasted demand, so that the huge potential of malaria vaccines to save lives is fulfilled. Together, they will work to accelerate a range of interventions, some of which are already underway, including: the rollout of technical assistance to support country application submissions and readiness for introductions; processes related to issuing policy recommendations and guidance so that the vaccines can be accessed as soon as possible after vaccine prequalification; support for implementing countries in registering new and existing vaccines. Gavi’s new co-financing policy (outlined above) also helps ensure the cost of malaria vaccines does not become an obstacle to demand materialisation.
Maximising the impacts of vaccines through innovation
A healthy vaccine market is underpinned by innovation. In the short- to medium-term, up to around 2028, advances are needed to maximise the potential impacts of the most advanced malaria vaccine products, RTS,S/AS01e and R21/Matrix-M. These could, for example, come in the form of products that meet WHO’s open vial policy, multi-dose vials, reductions in the number of doses required, or developments that better meet country programme needs or that lower costs. Gavi is seeking to identify and disseminate information to manufacturers about advances that would help existing and late-stage pipeline vaccines better meet country product preferences.
In September 2022 the WHO published updated Preferred Product Characteristics (PPCs) for malaria vaccines to incentivise and guide innovation. Beyond 2028, the Gavi Market Shaping Roadmap highlights that, when it comes to pipeline products, the Alliance has as an objective to support the development of at least one new licensed vaccine that either exceeds the WHO’s PPC malaria vaccine targets, is a much lower cost vaccine or one manufactured in Africa.
Gavi partners, including the Bill & Melinda Gates Foundation and the Coalition for Epidemic Preparedness Innovations (CEPI), directly support research and development and other forms of vaccine innovation. The Alliance’s market-shaping activities do so indirectly by helping to create an enabling environment for innovation in both existing and pipeline products.
Alliance partners aim to support malaria vaccine innovation. Regular engagement with existing and pipeline manufacturers and developers is important to inform decision-making relating to supply, demand, desired product attributes and programme updates. Gavi is also engaged in a process of learning lessons from the development of existing malaria vaccines and those for COVID-19, to inform how best to accelerate development, licensing, procurement and programme rollouts of future malaria and other vaccines.
Taking the fight to malaria
Despite progress in the last two decades, malaria remains a primary cause of childhood death and illness in sub-Saharan Africa. RTS,S/ASO1e, the first malaria vaccine to be recommended for use by the WHO, has already prevented illness and saved lives as a result of its use in the MVIP. A phase III trial carried out in seven African countries concluded that in children aged 5-17 months at first vaccination, four doses of RTS,S/ASO1e reduced cases of malaria by more than half during the first year following primary vaccination and by 39% at average follow-up of four years.
More recently, researchers who analysed data from a previous trial found RTS,S/ASO1e’s efficacy increased when children were vaccinated during the three months prior to the malaria transmission season.4 Phase 2b trial data for R21/Matrix M also show promising vaccine efficacy when delivered prior to the high malaria transmission season in areas with highly seasonal malaria transmission.5 While it may appear to have modest efficacy, given that there were 247 million cases of the disease in 2021, the impact of RTS,S and other malaria vaccines is expected to be huge. One life could be saved for every 200 children vaccinated. Its inclusion in routine childhood immunisation programmes could make it the third most impactful Gavi vaccine in terms of lives saved.
Malaria vaccines will provide countries with a critical new tool, complementing existing interventions to prevent and treat malaria. Insecticide-treated bed nets do save lives, but not everyone has access to them. They may be impractical to sleep under in some circumstances and they need re-treating. The mosquitoes that spread malaria can build up resistance to insecticides and antimalarial drugs. The MVIP pilot evaluations found that RTS,S/AS01e reached two-thirds of children that don’t normally sleep under bed nets, and, among those that did do so, immunisation did not discourage them from continuing to use them. Furthermore, even in areas with good bednet coverage, vaccine introduction resulted in a drop in severe malaria hospitalisations and death, showing the value of layering malaria prevention tools.
Researchers at Imperial College London and the Swiss Tropical and Public Health Institute carried out a modelling study in which they estimated RTS,S/ASO1e’s impact at different levels of coverage alongside existing malaria control interventions and treatments. They found RTS,S/AS01e could prevent the deaths of 24,000 children per year, assuming supply reaches 30 million doses per year. When the researchers assumed vaccine purchase prices of US$ 2, US$ 5 and US$ 10 per dose and took into account estimates of reduced case numbers, deaths and disability-adjusted life years, RTS,S/AS01e was found to be cost-effective at all three prices. Additional malaria vaccines are also expected to be cost-effective, with additional studies on cost-effectiveness planned to be conducted soon.
It took more than 30 years to develop the world’s first malaria vaccine. While its efficacy against malaria is relatively modest, the vaccine’s public health impact to reduce child illness and death from malaria is substantial. As the first vaccine to be approved for use against a human parasitic disease, it is a major achievement. Given that progress against malaria has stalled since 2015, malaria vaccines, starting with RTS,S/AS01e and subsequent malaria vaccines including R21, have the potential to reverse the recent uptick in cases, and drive both illness and deaths down significantly in the years to come.
References
- https://www.jci.org/articles/view/156588
- The Board has requested this special co-financing approach for malaria vaccines be revisited no later than 2027.
- In 2023, countries become eligible for Gavi support if their most recent gross national income (GNI) per capita was less than or equal to US$ 1,730 (according to the latest World Bank data published in July each year). Countries whose latest GNI per capita, or average over the past three years, falls below the threshold are classified as either initial self-financing (GNI per capita under the World Bank’s low-income country threshold) or in preparatory transition (above the World Bank’s low-income country threshold). These countries are eligible to apply for new vaccine support or health system and immunisation strengthening (HSIS) support from Gavi. For 2023, the eligibility threshold to enter the accelerated transition phase will be set at US$ 1,730 for both the latest
and the average GNI per capita over the past three years. - https://www.thelancet.com/journals/langlo/article/PIIS2214-109X(22)00416-8/fulltext
- https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(22)00442-X/fulltext