GAVI role in ever-growing demand for pentavalent
Pentavalent vaccine is administered at Freetown health clinic in Sierra Leone. Copyright: GAVI/2009/Olivier Asselin.
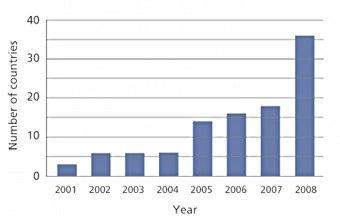
Number of countries eligible for GAVI support that have introduced pentavalent vaccine.
Source: GAVI Alliance, 2008
GAVI and its partners have played a key role in shaping the market conditions that have led to ever-growing demand for the pentavalent vaccine, which offers protection from diphtheria-tetanus-pertussis, Haemophilus influenzae type b (Hib) and hepatitis B (hepB).
By 2015, the Alliance aims to support the immunisation of an additional 230 million children with the five-in-one vaccine.
Building demand
GAVI support for pentavalent has laid the foundations for the increased demand giving countries and manufacturers confidence to include pentavalent in their long-term plans.
In July 2004, the GAVI Board established the Hib Task Force to explore how the Alliance could best support countries to make evidence-based decisions on the introduction of the Hib vaccine - a core component of pentavalent.
On the recommendation of the Task Force, in 2005, the Board allocated a four-year US$ 37 million grant to set up the Hib Initiative to catalyse the vaccine's uptake.
By bringing together the knowledge and expertise of the Johns Hopkins Bloomberg School of Public Health, the London School of Hygiene & Tropical Medicine, and the Centers for Disease Control and Prevention (CDC), the Hib Initiative has used a combination of collecting and disseminating existing data, research and advocacy to help countries build a case for adopting the Hib vaccine.
In only one year, the number of countries eligible for GAVI pentavalent support that introduced the vaccine rose from 18 (end 2007), to 36 (end 2008). By the end of 2010, 61 GAVI-eligible countries had been approved for funding for pentavalent vaccine, 59 of which had already introduced the vaccine.
In 2010, close to 28 million children were immunised with pentavalent vaccine with GAVI support
... and supply
At the same time, GAVI's subsidisation of pentavalent for countries interested in introducing vaccines against Hib and hepB has encouraged manufacturers to invest in developing new versions of the conjugate vaccine - a trend which is expected to further reduce vaccine prices and increase the long-term sustainability.
It is no coincidence that the number of WHO prequalified pentavalent vaccines has risen since 2005 - the year when both developing countries and manufacturers were reassured that GAVI's initial five-year life cycle would be extended.
The International Finance Facility for Immunisation (IFFIm) injected US$ 181 million into buying or securing the supply of pentavalent vaccine in 2006-2007 through GAVI. The long-term predictability and large-scale nature of GAVI support gives countries and manufacturers confidence to include pentavalent in their long-term plans.
Market-shaping
The growth in predictable demand supported by GAVI funding has created the sustainable market required to attract manufacturers, create competition and lower prices.