Contents
Benefits of the hexavalent programme
Gavi vaccine and programme funding for hexavalent
Documents
Hexavalent switch form
Gavi support guidelines
Contact
Interested Gavi-eligible countries should contact their Senior Country Manager. For general inquires, please email info@gavi.org with the words "Hexavalent vaccine" in the Subject line of your email.
Share
Eligible countries may apply for hexavalent vaccine
Programme update
Starting 1 December 2023, countries eligible for Gavi support can apply to switch to a whole-cell pertussis (wP) hexavalent vaccine (hexavalent) – a six-in-one vaccine that combines the pentavalent vaccine (diphtheria, tetanus, whole-cell pertussis [DTwP], hepatitis B and Haemophilus influenzae type b) with inactivated polio vaccine (IPV).
Hexavalent vaccine is expected to help countries deliver protection against all six diseases more efficiently and cost-effectively – as well as reduce programmatic challenges due to multiple injections, among other anticipated programmatic benefits.
Countries can choose to switch to hexavalent or continue using pentavalent and IPV. Vaccine Alliance partners can assist countries to assess the financial, logistical and programmatic implications of a switch to hexavalent to determine which option is most suitable for their context. Countries may also be eligible for operational support to introduce hexavalent vaccine.
Countries will need to continue using bivalent oral polio vaccine (bOPV) in their routine immunisation programmes until global cessation. Hexavalent contains IPV but not bOPV, and both are needed.
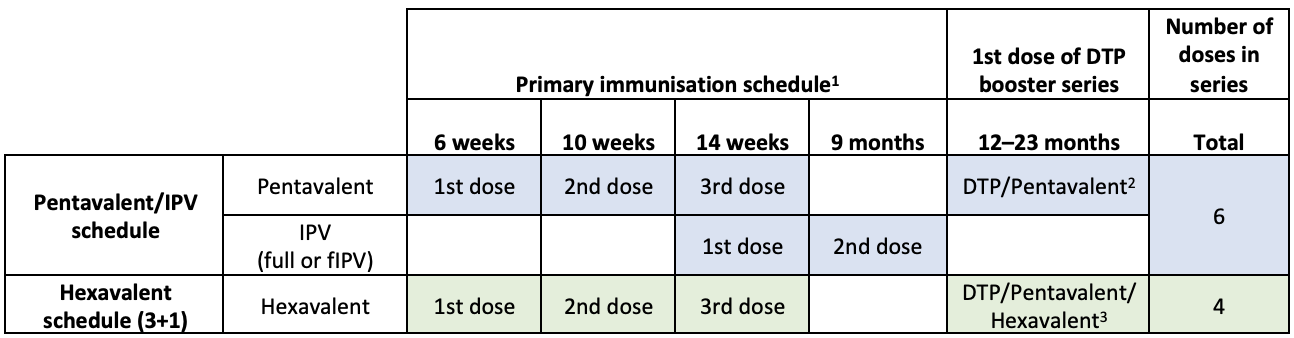
Hexavalent schedule
Starting from 6 weeks of age (minimum), the World Health Organization (WHO) recommends a hexavalent vaccine schedule of 3 doses with a minimum interval of 4 weeks between doses, and a booster dose given at least 6 months after the third dose. Illustrative schedules are shown below. Hexavalent switch form in English here and in French here; Gavi support guidelines here.
Benefits of the hexavalent programme
Programmatic efficiency
- Reduces number of injections: By combining pentavalent and IPV in the same vaccine, it reduces the number of injections and eases the burden on children, caregivers and health care workers – particularly in the context of vaccine hesitancy. This may also make it easier to reach zero-dose and under-immunised children.
- Reduces costs: Fewer doses administered lead to cost savings on syringes, safety boxes, cold chain, in-country transportation, associated energy consumption and labour.
Support for polio eradication
- Facilitates IPV coverage: Combining IPV with pentavalent increases opportunities for under-immunised children to receive sufficient IPV doses.
- Facilitates integration of IPV: It is anticipated that IPV will remain in routine immunisation well into the post-certification era. Providing IPV as part of a combination vaccine with other core antigens ensures streamlined integration into national immunisation programmes.
Strengthened immunisation programme
- Reinforces the second year of life (2YL) contact: The fourth dose of hexavalent, when given at 12-23 months of age, adds to strengthening the 2YL contact alongside other vaccines delivered at this time (e.g. second dose of measles-containing vaccine, malaria vaccine).
- Serves as the first DTP booster dose: Although recommended by WHO, many countries are not yet providing DTP-containing vaccine boosters. To support a life-course approach to vaccination, Gavi has opened a funding window for introducing DTP-containing vaccine boosters. The hexavalent booster aligns with and can serve as the first dose in the recommended DTP-containing vaccine booster series (2YL, 4–7 years and 9–15 years).
Gavi vaccine and programme funding for hexavalent
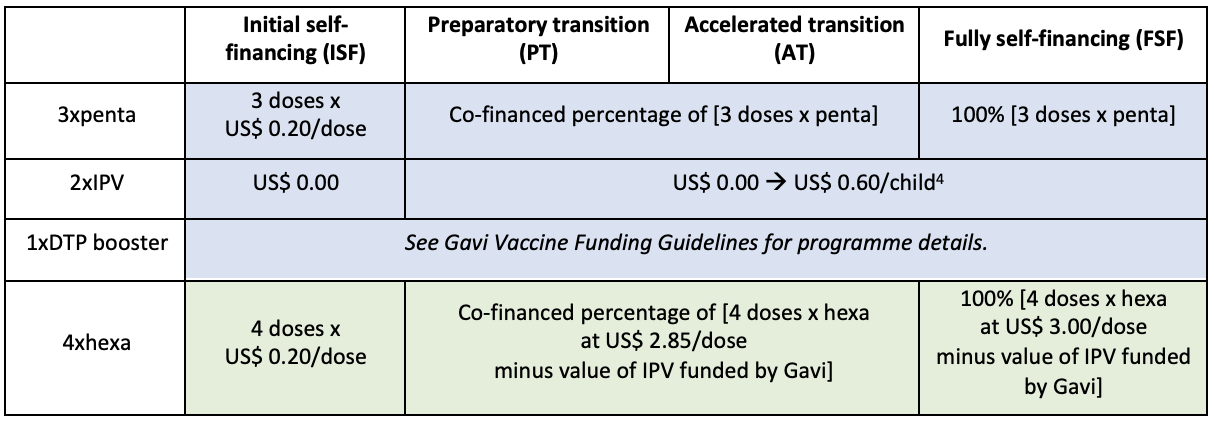
- In 2024, countries eligible for Gavi support will have gradual access to hexavalent vaccine at the price of US$ 2.85 per dose. Fully self-financing countries and countries eligible for Gavi support under the Middle-Income Countries (MICs) Approach can procure the vaccine through UNICEF at the price of US$ 3.00 per dose (source: UNICEF).
- Countries that decide to switch to hexavalent will have to co-finance this vaccine based on the regular Gavi co-financing policy, and they will continue to benefit from the IPV co-financing exemptions as described in the table below. Fully self-financing countries can request a subsidy for the purchase of hexavalent vaccines, equivalent to their IPV eligibility.
- Countries may be eligible for operational support to introduce the hexavalent vaccine. For more information, please refer to the Q3 2023 Vaccine Funding Guidelines.
Co-financed share by Gavi-eligible countries
Special considerations for switching to hexavalent
- The feasibility and financial implications (including savings) of switching to hexavalent: Countries may pay more in vaccine costs for the hexavalent 4-dose series than for the current 3-dose pentavalent + 2-dose IPV, but they would benefit from programme cost savings in delivery (e.g. reduced injections, health care worker labour).
- 10-dose presentation: Initially, hexavalent will only be available to Gavi-eligible countries in a 10-dose presentation. Countries will need to consider the programmatic implications (e.g. cold chain, wastage) of using 10-dose vials if they are currently using a 1-dose presentation for their pentavalent product.
- Planning and implementation logistics: Countries will need to switch from pentavalent to hexavalent; phase out of standalone IPV; and introduce a hexavalent booster during the 2YL contact (for most countries).
- Introduction of a second dose of IPV (IPV2): WHO recommends that countries introduce IPV2; those that have not done so yet are encouraged to introduce hexavalent or IPV2 as a matter of urgency.
- Programmatic shifts to a 2YL contact: Tailored interventions, such as training and communications, will be necessary to ensure uptake and high coverage of the fourth hexavalent dose.

