Insight paper
April 2025
Contents
1. The value and potential of malaria vaccines
2. Vaccines as part of a broader antimalaria strategy
3. Rolling out malaria vaccines: successes and challenges
4. Financing malaria vaccine roll-outs
Share
© The Gavi Alliance. All rights reserved. This publication may be freely reviewed, quoted, reproduced or translated, in part or in full, provided the source is acknowledged.
The material in this publication does not express any opinion whatsoever on the part of Gavi, the Vaccine Alliance concerning the legal status of any country, territory, city or area or its authorities, or of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by Gavi, the Vaccine Alliance.
Please contact media@gavi.org with any questions about use.
Executive summary
After decades as a promise for the future, the first vaccine to prevent malaria was recommended for use by the World Health Organization (WHO) in 2021, followed by a second in 2023. Today, they are in demand, in use and saving lives. They are urgently needed.
In 2023, malaria is estimated to have caused nearly 600,000 deaths, mostly among children in the WHO African Region. The RTS,S and R21 vaccines have been shown to reduce clinical malaria cases by more than 50% in the 12 months after three initial doses. More than 24 million doses have been delivered to 20 countries. Rolling out the vaccines further in these countries and others presents a major opportunity to save and improve lives in at-risk and vulnerable communities. It is an opportunity the world must urgently seize by supporting the hopes, efforts and ambitions of affected countries to defeat this disease once and for all.
This insight paper provides an update on malaria vaccine roll-outs, the first of which began in January 2024, and an analysis of some of the successes and challenges faced by those leading them. Additionally, this paper summarises some of the ways Gavi, the Vaccine Alliance is helping to increase access to malaria vaccines and outlines Gavi’s recommendations to stakeholders working to maximise the impact of the vaccines.
Gavi is calling for decision-makers in countries with moderate and high transmission of malaria to:
- consider investing in malaria vaccine introduction as part of malaria control programmes, if they have not already done so;
- integrate malaria vaccines and related activities into malaria prevention and control programmes;
- engage communities and confront vaccine misinformation; and
- make use of peer-to-peer learning opportunities to maximise malaria vaccine impact.
Gavi is calling for global and regional health organisations to:
- strengthen coordination with other organisations and stakeholders and reduce the impacts of malaria;
- support the integration of malaria vaccines into malaria control programmes;
- listen to country voices about the need for malaria vaccines; and
- support further research on malaria vaccines.
Gavi is calling for donors to:
- provide financial support for malaria vaccine programmes to save lives, protect health, improve global health security, encourage affected countries to invest in malaria control; and to boost the global economy.
Introduction
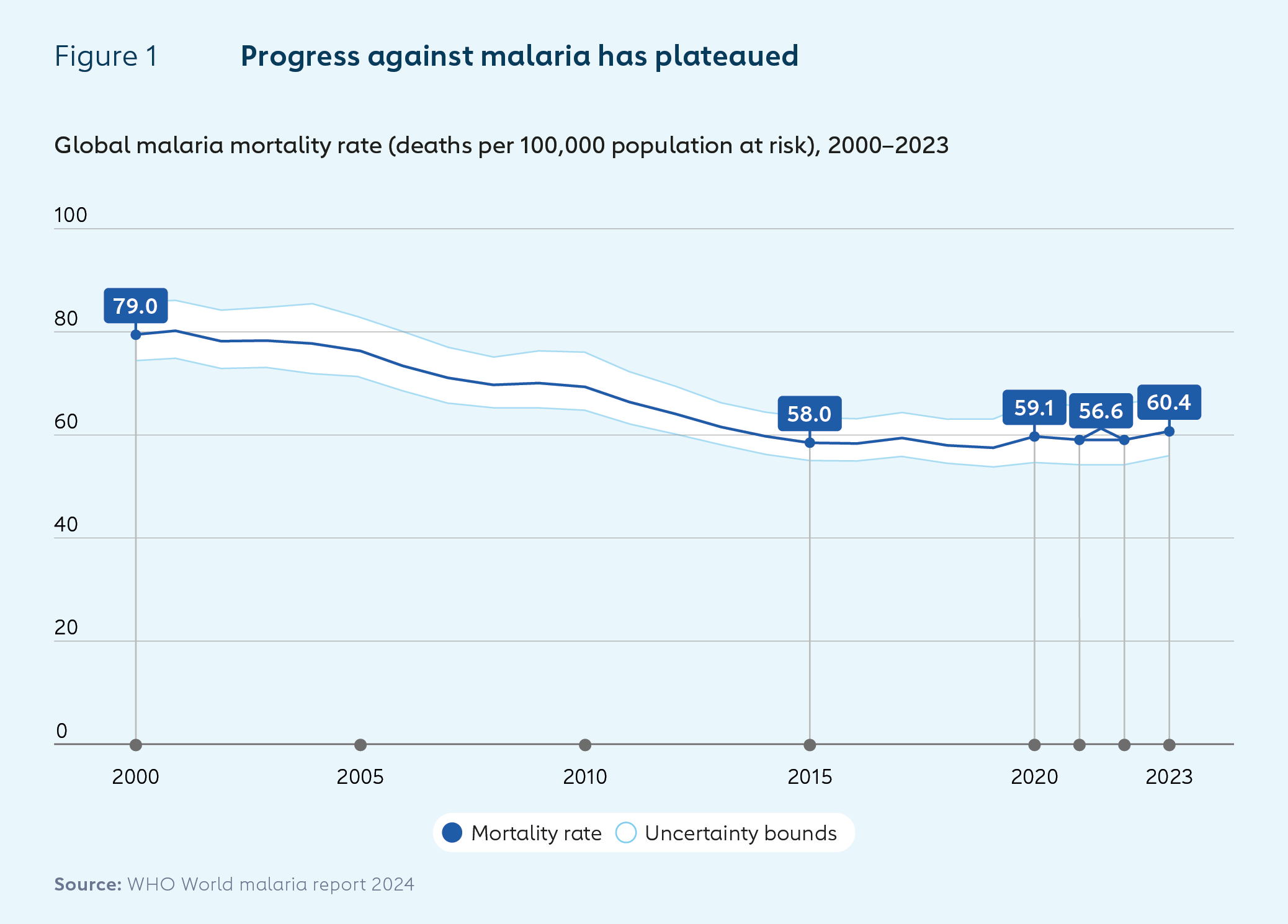
Malaria is an infectious disease caused by Plasmodium parasites, which are spread to people through the bites of infected female Anopheles mosquitoes. In 2023, the number of estimated cases worldwide rose by 11 million to 263 million year on year, while deaths fell slightly from an estimated 600,000 to 597,000.1
Case incidence as a proportion of the global at-risk population was higher in 2023 than in 2019, highlighting the continued effects of the disruption of health services caused by the COVID-19 pandemic. African countries bear most of the burden of malaria, especially among children. Countries in the WHO African Region accounted for 94% of cases and 95% of deaths in 2023.
While malaria is the third deadliest infectious disease worldwide, its impacts extend well beyond its death toll. The parasites that cause it can lie dormant in the liver for years before spreading to the blood and causing symptoms. These can include extreme tiredness, convulsions, breathing difficulties, abnormal bleeding, organ failures, cognitive impairment and visual problems.
The costs of malaria come in various forms. First, there is the financial investment needed to prevent and control the disease. Then, there are the social and economic effects of failing to do so: time spent ill; education missed; the cost of caring for the sick; lost wages; and an annual US$ 12 billion in lost productivity worldwide.2
The spread of malaria in regions in which it is endemic increases the number of travellers with the disease returning home to non-endemic countries. While most of the burden of malaria falls on lower-income countries, the costs of failing to prevent and control the disease therefore extend to higher-income countries too. Approximately 2,000 cases of malaria are reported annually in the United States of America, mostly in travellers. In 2023, the mean cost of treating a patient hospitalised with malaria in the United States was estimated at more than US$ 20,000.
Traditional malaria prevention and treatment strategies such as vector control measures and antimalaria medicines have helped to reduce deaths significantly since the year 2000. Global deaths attributable to the disease fell by around 40% between then and 2019. The rate of decline in deaths per 1,000 population at risk slowed from 2013. And beginning in 2020, disruptions caused by the COVID-19 pandemic caused an increase in cases and deaths.
The roll-outs of RTS,S and R21, the first two vaccines against malaria, have the potential to further reduce deaths and disease burden, particularly now – as scientists are warning that climate change is driving up malaria transmission rates and widening its geographical distribution.
To prevent financial constraints becoming a barrier to uptake, Gavi has developed an exceptional approach to financing malaria vaccines. Gavi support can cover the funding for 85% of children targeted in moderate- and high-transmission areas. The Vaccine Alliance is currently engaged in a review of its eligibility, transition and co-financing policies.
This insight paper provides an update on malaria vaccine roll-outs, and an analysis of the successes and challenges so far. Additionally, it summarises some of the ways Gavi is supporting countries to increase access to malaria vaccines; and outlines Gavi’s recommendations to maximise the impact of malaria vaccines.
1. The value and potential of malaria vaccines
The World Health Organization (WHO)’s recommendations of the use of RTS,S, the first vaccine for a human parasitic disease, in 2021, and then of R21 two years later, to prevent Plasmodium falciparum malaria in children living in endemic areas, were significant milestones in the fight against the disease.
Both vaccines have been shown to be safe and to reduce clinical malaria cases by more than 50% during the 12 months after three initial doses. Fourth doses prolong efficacy, and trials suggest both vaccines can prevent close to three quarters of cases when provided prior to the onset of the rainy season in areas of high seasonal transmission.3,4
Studies show that introducing malaria vaccines into routine immunisation programmes has had no negative impact on the uptake of other vaccines, use of insecticide-treated bed nets or care-seeking behaviours for fever.
Trials of RTS,S and R21 have varied in their study design, the lengths of follow-up, approaches to giving the vaccine and in the transmission rates of the areas tested, and none have directly compared the efficacies of the two vaccines. WHO has concluded that there is currently no evidence to suggest that one of the two vaccines outperforms the other.
Gavi has forecast that fully immunising around 50 million children against malaria during 2026–2030 could save more than 170,000 lives.5

2. Vaccines as part of a broader antimalaria strategy
As with some other vaccines, the efficacy of malaria vaccines declines over time. It has been estimated (see Figure 2 above) that malaria vaccines can achieve the greatest impact when they are used alongside other interventions, including insecticide-treated bed nets, indoor residual spraying, seasonal malaria chemoprevention, and effective case management and treatment. 6,7,8,9
To maximise impact, National Malaria Control Programmes and National Immunisation Programmes in each country must work together to assess the burden of malaria, identify priority geographical areas to target with the vaccine and coordinate to support each other’s activities.
Information, education and communication materials related to the vaccine should reference other antimalaria interventions, and vice versa.
There are also opportunities for integration of multiple interventions across service delivery. Some countries are, for example, providing insecticide-treated bed nets alongside the fourth malaria vaccine dose in the second year of life.
Other countries with highly seasonal malaria are planning to coordinate communication campaigns for the fourth vaccine dose and seasonal chemoprevention so that both can be provided prior to the high malaria transmission season.
Data monitoring is another area in which more can be achieved through coordinated activity. Health workers can, for example, check vaccination status when they are administering seasonal malaria chemoprevention.
Gavi supports countries to build strong, equitable, sustainable immunisation programmes through its focus on health systems and immunisation strengthening (HSIS). Gavi provides HSIS funding to strengthen delivery of all routine vaccines including malaria with investments in key areas including: human resources; service delivery; demand generation; community engagement; supply chains; data collection, analysis and use; programme management; and sustainable financing.
These interventions can be leveraged to support malaria vaccine roll-outs, while also presenting significant opportunities for broader immunisation and health benefits. Additionally, Gavi offers support through its Equity Accelerator Fund as part of its continued commitment to reaching zero-dose children and other vulnerable and under-served populations.
Case study
Integrating malaria vaccination into broader malaria prevention and control measures in Ghana
More than 11,000 people lost their lives to malaria in Ghana in 2023.10
The country contributed significantly to global understanding of the RTS,S vaccine, having been involved in a phase 2 trial from 2006, a 2009–2014 phase 3 trial and the country-led pilot, the Malaria Vaccine Implementation Programme (MVIP), from 2019. Ghana also played a key role in building support for malaria vaccines within the Gavi Board.
It successfully introduced RTS,S in 42 of its 261 districts beginning in 2019 before adding another 51 districts in February 2023, followed by the roll-out of R21 in 43 districts in September 2024.11
Ghana’s National Malaria Control Programme and National Immunisation Programme have therefore long understood the benefits of working together to integrate malaria vaccination, both with other antimalaria interventions and with the delivery of other vaccines. Most bed net distribution occurs when mothers and other caregivers bring their children for their routine immunisation sessions.
Health workers and others involved in malaria prevention and control, from community to national levels, are trained to implement integrated approaches to malaria control. In community health facilities, it is often the same health workforce that delivers malaria and other childhood vaccinations.
This coordination of work and resources also extends to public information and education campaigns, through channels including television, radio, social media, leaflets and text messaging. Posters used to publicise malaria vaccination highlight the importance of continuing to use other malaria prevention tools and the importance of seeking help for children with symptoms, for example.
3. Rolling out malaria vaccines: successes and challenges
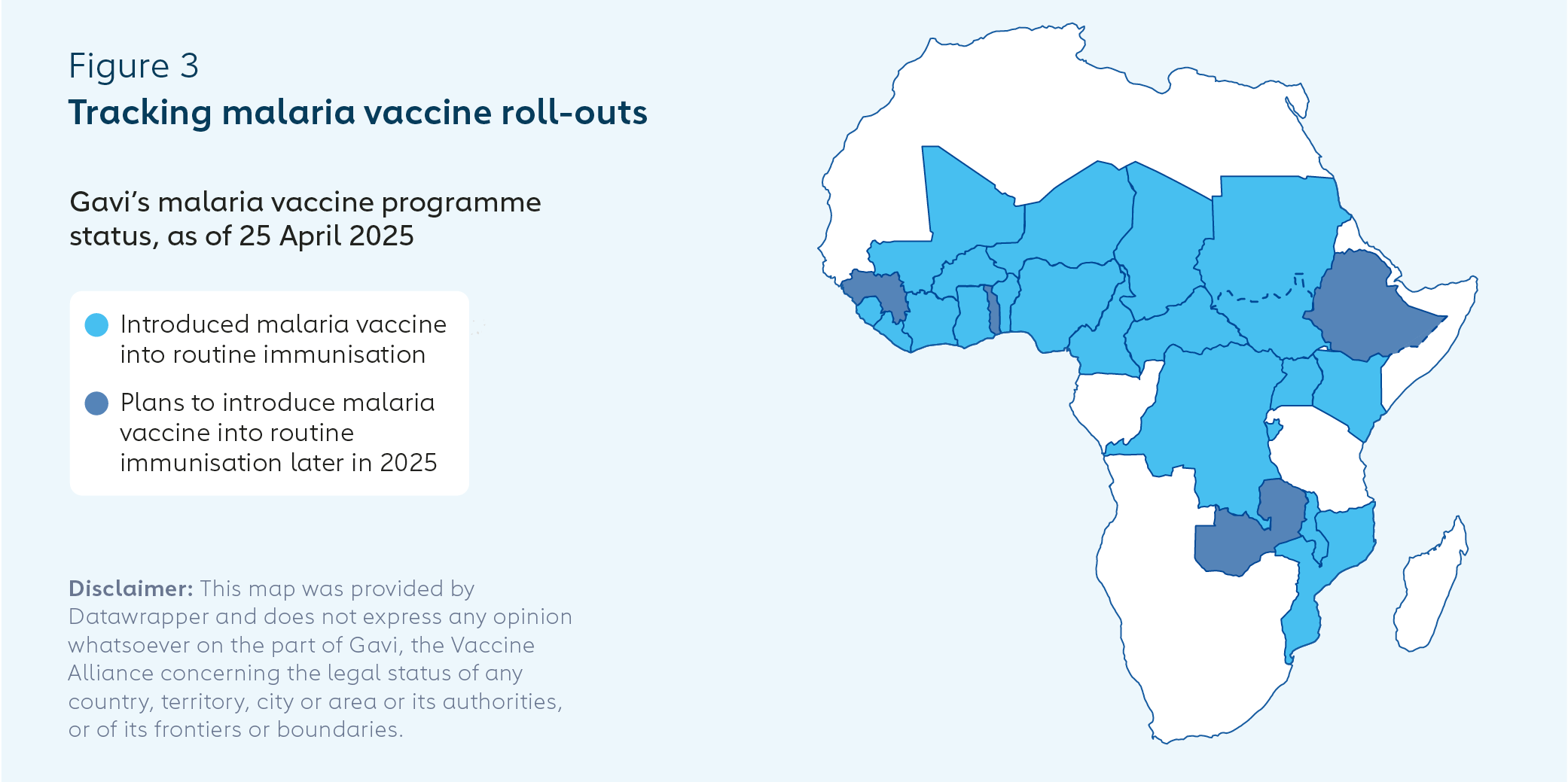
Gavi is working with more than 30 countries that have expressed interest in introducing malaria vaccines. At the time of writing (April 2025), 25 countries had been approved for Gavi funding to support malaria vaccine introductions, of which 13 had been approved for further malaria vaccine scale-up. A total of 20 countries had introduced malaria vaccines into their routine immunisation programmes, and a further 4 were planning to do so by the end of 2025.
More than 6 million doses of malaria vaccine were delivered to Ghana, Kenya and Malawi as part of the MVIP from 2019–2023, protecting about 2 million children.12 Close to 10 million doses were delivered to 17 total countries in 2024, protecting an estimated 5 million children.
Vaccine coverage and uptake takes time to build up. Ghana, Kenya and Malawi collectively increased their first-dose malaria vaccine coverage from 75% to 84% from 2020–2023, and their third dose coverage from 66% to 78% over the same period.13
Malaria vaccine delivery strategies vary according to local priorities and contexts. Transmission occurs year-round in warmer regions closer to the equator and is more seasonal in cooler areas because the growth cycle of the Plasmodium falciparum parasite is temperature-dependent.
Countries may adopt: (1) an age-based malaria vaccination strategy, providing four doses throughout the year for children from the age of about five months; (2) a seasonal approach of three doses monthly, ending just before the start of the high transmission season, followed by a fourth dose 12–18 months later – just before the period of high transmission; or (3) a hybrid of the two.
In alignment with WHO guidelines, a fifth dose can be provided one year after dose 4, in areas with high seasonal transmission or where malaria risk remains high for children.
There is also some flexibility in the recommended timing of doses to enable countries to take into account factors such as seasonal transmission and operational considerations, such as grouping malaria vaccination with other routine vaccinations to maximise coverage and public health impact.
Each new malaria vaccine introduction will generate potentially useful new data and provide opportunities for countries to learn how to improve impact based on different vaccination schedules and strategies.
Recent malaria vaccine introductions
Three countries launched or were due to launch malaria vaccine introductions in March and April 2025. Burundi became the 18th country to introduce malaria vaccine into its routine immunisation programme on 17 March.
The largest malaria vaccine introduction to date began on 2 April, in Uganda, with initial plans to target 1.1 million children with four doses at 6, 7, 8 and 18 months of age. Apac District, in northern Uganda, is reported to have the highest number of mosquito bites per person globally (over 1,500 bites per person annually).
On 25 April (World Malaria Day), Mali launched the first age-based and seasonal hybrid approach – providing the first three doses monthly throughout the year, dependent on age, followed by fourth and fifth doses just before the start of the high transmission season.
Malaria vaccine roll-outs are progressing successfully. There are, however, challenges for governments, health workers and civil society organisations (CSOs) working to maximise the impact of the vaccines.
While countries contributed a record US$ 215 million towards the co-financing of Gavi-supported vaccines in 2023, ongoing global and regional economic challenges are straining the fiscal capacity of many countries, potentially undermining their ability to invest further in strengthening immunisation programmes. More than half of low-income countries are either experiencing, or at a high risk of, debt distress. Middle-income countries, including former Gavi-supported countries, are also impacted by the difficult economic climate.
We have good malaria vaccine uptake and coverage in Ghana, however the country has been through some economic hardships following the COVID pandemic. It has entered the Gavi accelerated transition phase, which means the contribution it must make for vaccine doses is higher than before. Gavi is funding 85% of the need in areas with moderate to high malaria transmission, so Ghana must find the resources to meet its co-financing payments, but also to fill the gap if it wants to reach the other 15%. Beyond procuring the vaccine, there are extra operational costs linked to human resources, communication, transport, data recording tools and other logistical requirements.
John Bawa
Director of Malaria Vaccine Implementation, PATH, Ghana
Gavi is working hard to ensure these economic and financial issues do not prevent malaria vaccines from achieving their potential (see “Financing malaria vaccine roll-outs” below). Elsewhere, misinformation, politics, conflict, workforce issues, geography and even the weather can cause challenges to rolling out malaria vaccines.
On 2 April 2025, Uganda’s Ministry of Health, with support from Gavi, UNICEF, WHO, PATH and CHAI, launched the largest malaria vaccine introduction to date.
Apart from the problems we had with misinformation (see “Confronting vaccine misinformation through community engagement in Cameroon” below), we faced some service provision bottlenecks. The ongoing social and political crisis in the northwest and southwest of the country makes it difficult for government officials to operate. As the EPI, we can sometimes go in to provide vaccines, but we have to be very careful and wait for security windows. In some remote areas where the security situation is okay, we have health workforce shortages which limit our capacity to reach people. Also, sometimes the supply plan does not meet demand. In November and December [2024], farmers in some areas were on holiday, and there was no rain to make access difficult, leading to a surge in demand and stock-outs in many health facilities.
Tchokfe Shalom Ndula
Permanent Secretary of the Expanded Programme on Immunization (EPI), Cameroon
During 2024, WHO, PATH and other partners organised a series of peer-learning workshops at which countries shared best practices from pilot implementations and recent introductions. These were designed to equip countries with tools and strategies to drive effective malaria vaccine roll-outs. Further peer-learning activities are planned.
The TechNet network provides a useful summary of current global recommendations, evidence and programmatic considerations for malaria vaccine introduction into national immunisation programmes. The WHO Regional Office for Africa provides weekly webinars to showcase country examples of malaria vaccine introductions.
Case study
Confronting vaccine misinformation through community engagement in Cameroon
WHO estimates that Cameroon saw more than 7.3 million malaria cases and 11,600 deaths in 2023.14 The country became the first to add malaria vaccine to its routine immunisation programme on 22 January 2024, following the MVIP pilots in Ghana, Kenya and Malawi. The initial roll-out covered the 42 districts at greatest risk. Coverage rates at the end of 2024 were 65% for the first dose and 48% for three doses.
The government faced significant challenges in the early stages of the vaccine introduction due to misinformation and vaccine hesitancy. This is believed to have been exacerbated by a decline in trust in public policy that grew during the roll-out of COVID-19 vaccines.
Cameroon mounted a successful response to malaria vaccine misinformation. An emergency plan for risk communication was devised by CSOs, community and religious leaders, the EPI and global health partners. Technical experts, including Dr Rose Leke, the renowned Cameroonian immunologist, were recruited to explain the advantages of malaria vaccination via traditional and digital media.
Members of the public were invited to engage in dialogue at community meetings that included traditional and religious leaders. These successful community engagement efforts have led to high levels of public acceptance of the vaccine and growing demand from parents.
4. Financing malaria vaccine roll-outs
Gavi seeks to expand access to life-saving immunisation in lower-income countries while emphasising country ownership and domestic financing of vaccines and their delivery. The co-financing of vaccines by countries helps ensure the sustainability of immunisation programmes and is at the heart of the Gavi’s catalytic funding model. The record total contribution of countries towards the co-financing of Gavi-supported vaccines in 2023 (see “Rolling out malaria vaccines: successes and challenges” above) represents an important achievement.
However, many countries are still recovering from the COVID-19 pandemic, with financial constraints causing budget pressures, especially in low- and middle-income countries – for which the price of malaria vaccines remains relatively high.
In recognition of these challenges, in December 2022, the Gavi Board approved an exceptional co-financing approach for malaria vaccines to prevent financial constraints becoming a barrier to uptake. Gavi provides funding for vaccines for 85% of children targeted in moderate- and high-transmission areas to ensure effective use of resources. The Vaccine Alliance is engaged in a review of its eligibility, transition and co-financing policies to ensure these align with its goals during Gavi’s next strategic period (2026–2030).
In accordance with the Lusaka Agenda, Gavi is committed to working with international partners to ensure complementarity and to better support stronger, more integrated country-level planning that responds to national health and immunisation strategies. In March 2024, ministers of health from African countries with the highest malaria burdens signed a declaration committing to stronger leadership and increased domestic funding to combat the disease.
The Vaccine Alliance and the Global Fund to Fight AIDS, Tuberculosis and Malaria are working together to provide funding for malaria programmes and the health systems that support them. The Global Fund is focused on strengthening prevention and control efforts, while Gavi supports the procurement, roll-out and delivery of malaria vaccines. In its 2026–2030 strategic period, Gavi plans to invest more than US$ 1.1 billion in malaria vaccination programmes, helping vaccinate at least 50 million children.15
As part of their collaboration, Gavi and the Global Fund have published a joint guidance document that sets out malaria vaccine programme areas that each organisation can support and their co-financing requirements.
Conclusion
Gavi’s key recommendations to stakeholders working to maximise the impacts of malaria vaccines are as follows:
For decision-makers in countries with moderate and high transmission of malaria
1. Consider investing in malaria vaccine introduction
There are now two safe and effective malaria vaccines. Introducing them in at-risk countries in accordance with national plans and priorities has significant potential to save lives, reduce illness and bring a host of societal, economic and other benefits.
2. Integrate malaria vaccines and related activities into broader malaria prevention and control programmes
Malaria vaccines are independently effective; however, the greatest impacts can be achieved when they are used alongside other existing interventions. The addition of malaria vaccines to antimalaria programmes opens opportunities to maximise impact by coordinating activities in areas including public information, education, service delivery, health worker training and data monitoring.
3. Engage communities and confront vaccine misinformation
Early public information, education and community engagement through media and community meetings can build trust and vaccine demand. Misinformation can be countered through coordinated responses involving CSOs, community leaders and technical experts.
4. Make use of peer-to-peer learning opportunities
Malaria vaccine introductions based on different vaccination schedules and strategies are generating important new data and learnings. Monitoring these and participating in peer-learning activities can help countries maximise vaccine impact.
For global and regional health organisations
1. Strengthen coordination with other organisations and stakeholders in the fight against malaria
The Malaria Vaccine Coordination Team (MVCT), co-chaired by Gavi and WHO, brings together stakeholders including the Global Fund, UNICEF, PATH, the World Bank, and the Africa Centres for Disease Control and Prevention (Africa CDC) to coordinate immunisation approaches. Further efforts are needed to achieve strategic and operational coherence.
2. Support the integration of malaria vaccines into malaria control programmes
Malaria vaccines are not silver bullets. They can best drive progress when they are used alongside insecticide-treated bed nets, indoor residual spraying, seasonal malaria chemoprevention, and effective case management and treatment.
3. Empower country voices to make decisions about malaria vaccines
Decisions on malaria vaccines must be made at country level. Global and regional health organisations should work to empower government representatives, parliamentarians, National Immunisation Technical Advisory Groups (NITAGs), CSOs and local communities to ensure the sustainability of malaria vaccine and other immunisation programmes.
4. Support further research on malaria vaccines
A global research agenda on malaria vaccines was published in 2024 covering themes including: safety; implementation; acceptability and demand; integration with other health interventions; impact and effectiveness; economics; and cost-effectiveness. Supporting such research can drive further gains against malaria.
For donors
1. Invest in malaria vaccines to save lives and improve health
The huge demand for malaria vaccine from parents in at-risk countries presents major opportunities for donors to prevent deaths, reduce the burden of illness and improve lives in low- and middle-income countries. Funding malaria vaccination programmes also encourages countries affected by the disease to invest in malaria control and protects global health security, while boosting the global economy in the long term.
References
- https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2024
- https://www.who.int/multi-media/details/malaria-vaccines(rts-s-as01-and-r21-matrix-m)-infographic--april-2024
- https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(23)02511-4/fulltext
- https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(23)00368-7/fulltext
- The forecast is a function of estimates of population size, disease burden and forecasted introduction; scale-up and coverage of vaccination and as a result is subject to change.
- https://media.path.org/documents/Product_info_fact_sheet_RTSS-R21_March_2024.pdf
- https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD000363.pub3/full
- https://www.nejm.org/doi/full/10.1056/NEJMoa2026330
- https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(23)00368-7/fulltext
- https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2024
- https://www.unicef.org/ghana/press-releases/ghana-expands-malaria-vaccine-rollout#:~:text=The%20expansion%20will%20begin%20on,disease%20epidemiology%20and%20resource%20availability
- https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2024
- https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2024
- https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2024
- https://www.gavi.org/news/document-library/2026-2030-gavi-investment-opportunity

