Gavi’s impact
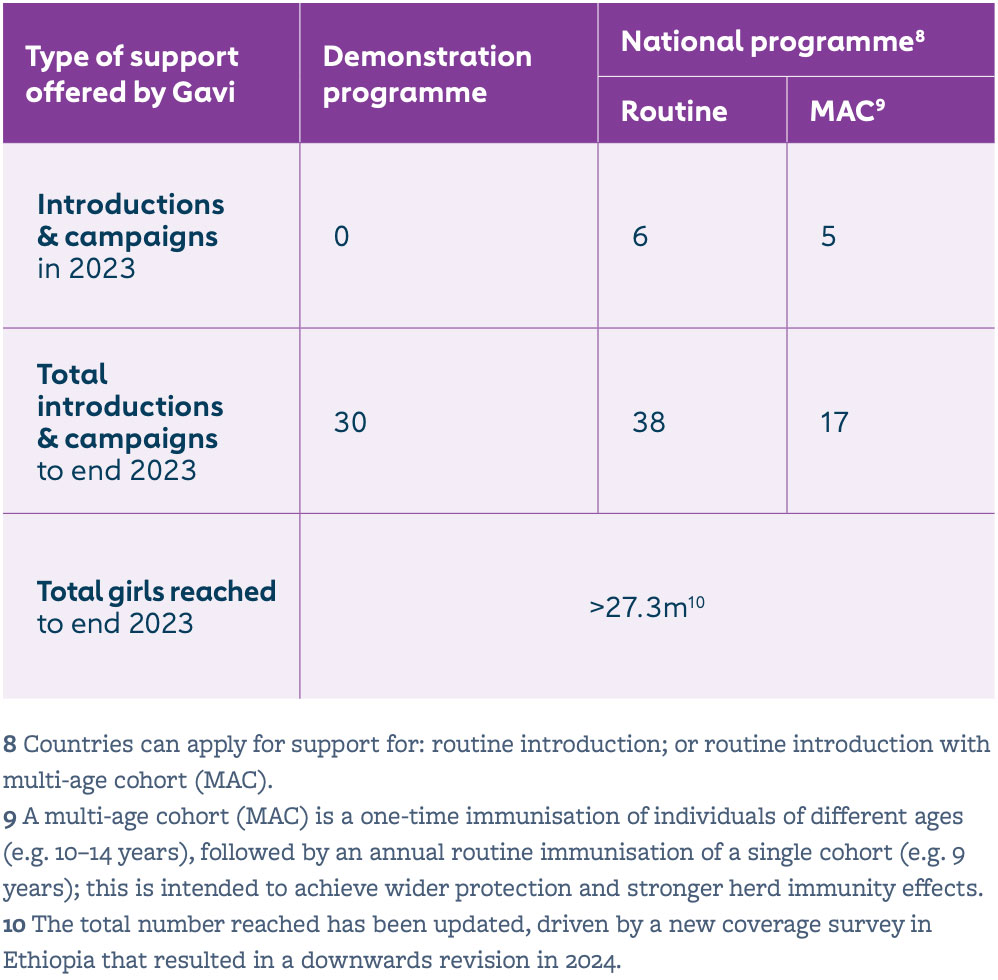
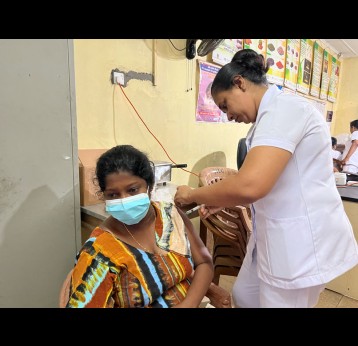
Human papillomavirus (HPV) vaccine coverage in the 57 lower-income countries supported by Gavi increased from 4% in 2019 to 8% in 2022 and 16% in 2023. This was largely driven by new countries introducing the vaccine into their national immunisation programmes. By end 2023, 38 countries had successfully launched their HPV vaccine national programme with Gavi support, fully immunising more than 27.3 million girls since 2014, which translates to over 605,000 future cervical cancer deaths averted with Gavi support.
In a WHO position paper released in December 2022, the Strategic Advisory Group of Experts on Immunization (SAGE) recommended a one- or two-dose schedule for HPV vaccine. A single dose schedule greatly simplifies delivery of the vaccine, as you only need to reach a girl one time for full protection. In response to this potential gamechanger for Gavi’s HPV vaccine programme – and to overcome the challenges and impact of the COVID-19 pandemic on HPV vaccine coverage – in December 2022, the Gavi Board approved the revitalisation of Gavi’s HPV vaccine programme with a US$ 600+ million investment through 2025. With additional funding in place, the Alliance has set an ambitious goal to reach over 86 million girls with HPV vaccine by end of 2025, aiming to avert over 1.4 million future deaths from cervical cancer.
The issue
Cervical cancer is the fourth most common cancer in women globally, causing more than 209,000 deaths in 2022 in lower-income countries. However, HPV is associated with over 90% of these cancers, and vaccination provides high protection against cervical cancer. HPV vaccine has among the highest impact of all Gavi-supported vaccines, with 17.4 deaths averted per 1,000 children vaccinated, and is one of the key interventions towards achieving the global strategy for cervical cancer elimination.
HPV vaccination is critical to reducing cervical cancer, especially in lower-income countries with a high disease burden and less developed cervical cancer screening and treatment programmes. It is also a bridge to improving women’s and girls’ health through increased touchpoints with health services, and therefore an opportunity to positively impact gender equity.
Gavi’s response
Historically, the high cost of HPV vaccine, limited supply and challenges of reaching adolescent girls to deliver immunisation have been barriers to introduction in lower-income countries. Gavi is working to bridge the equity gap by providing support for national HPV vaccine introductions and ensuring sustainable prices. Gavi supports routine HPV vaccine delivery to a single cohort of girls (e.g. aged 9 years); and a multi-age cohort (MAC) (e.g. aged 10–14) in the year of introduction.
By (1) accelerating quality introductions; (2) rapidly improving global and national coverage; and (3) generating long-term programmatic sustainability, the revitalisation will allow Gavi to dedicate additional funding to help countries and partners reach more girls than ever with this life-saving vaccine. Support available under the US$ 600+ million revitalisation initiative includes expanded funding for vaccine purchasing, introduction and campaign activity costs, and adoption of the single dose-schedule; and expanded technical assistance from partners at regional, national and local levels. Gavi-eligible countries can also utilise health system strengthening (HSS) funding to develop tailored approaches to improve and sustain HPV vaccine coverage.
Lastly, Gavi is investing in implementation research focused on: strengthening integration of services for adolescents; integration of cervical cancer elimination strategies; and strengthening measurement of HPV vaccine coverage.
Gavi currently procures three HPV vaccines that have been prequalified by WHO. After severe supply constraints starting in 2017, HPV vaccine supply outlook is improving – supported by rapid uptake of the single-dose schedule, ramped up production from suppliers and WHO prequalification of new HPV vaccine products.
Gender and HPV vaccine
Not only does HPV vaccine prevent a major cause of female mortality, HPV vaccine delivery is also a touchpoint between adolescent girls and health services which can act as a bridge to improving women’s and girls’ health across the life course. It is an opportunity to build adolescents’ knowledge and trust in the healthcare system; deliver other essential health and education services; and empower girls through involving them in programme design and social mobilisation activities.
Social and cultural norms, and the unequal status of women in many societies, can reduce the chances of children and adolescents being vaccinated, by preventing girls and their caregivers from accessing immunisation services. For example, school-based vaccination programmes may miss girls who are not able to attend school due to gender-related barriers. Therefore, Gavi is investing in innovative approaches to reach out-of-school girls, and to achieve high and equitable coverage.
FROM OTHER SITES