Gavi's impact
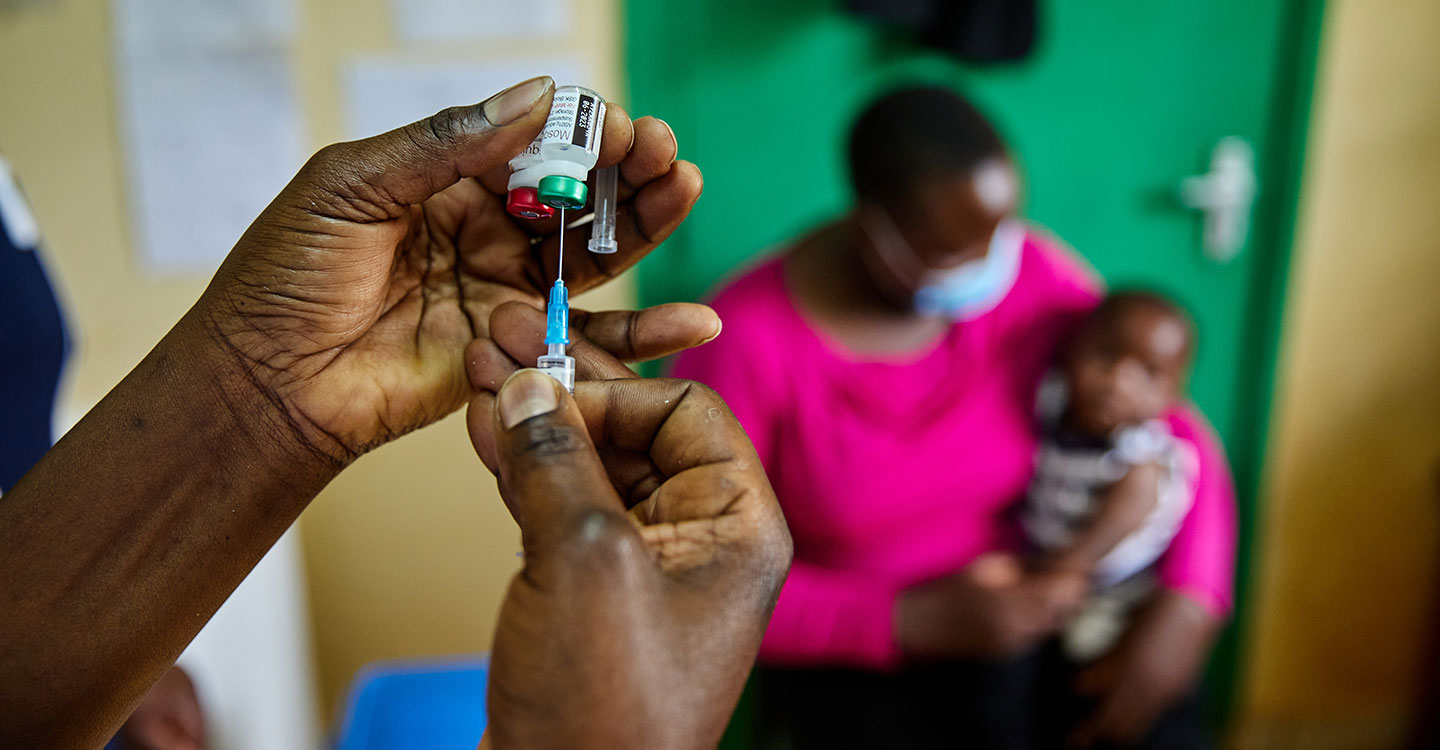
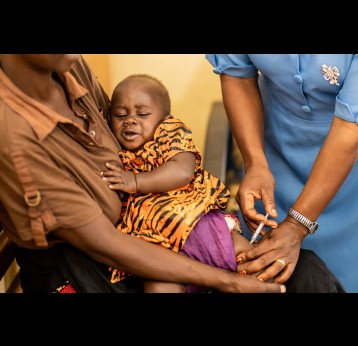
In December 2021, the Gavi Board made history by approving funding to support the roll-out of the world’s first malaria vaccine – nearly 35 years in development – in sub-Saharan Africa in 2022–2025. According to the World Health Organization (WHO), the vaccine is estimated to save 1 life for every 200 children vaccinated.
Consistent funding and investment are crucial to ensure this new, vital tool reaches equally all those who need it. As long as resources are available, Gavi pledges its continued commitment and support to the fight against one of Africa’s deadliest diseases.
The issue
Children aged under five years are at the greatest risk of dying from malaria and account for more than 75% of the global deaths from the disease. Unlike adults, young children have not had the opportunity to develop partial immunity through years of exposure, making them particularly at risk.
In 2023 alone, malaria caused 597,000 deaths globally, and 432,000 child deaths in Africa – highlighting the urgent need to protect our youngest and most vulnerable. The African region shoulders the heaviest malaria burden globally, accounting for about 94% of all cases and 95% of deaths, according to WHO data.
Gavi's response
Malaria vaccines
As of January 2026, over 39 million doses have been delivered to 25 endemic countries in Africa which have introduced malaria vaccines into routine immunisation with Gavi support:
- 1 country introduced the vaccine in 2026: Guinea-Bissau.
- 7 countries have introduced the vaccine in 2025: Burundi, Uganda, Mali, Guinea, Togo, Ethiopia and Zambia.
- 14 countries introduced the vaccine in 2024: Cameroon, Burkina Faso, Sierra Leone, Benin, Liberia, Côte d’Ivoire, South Sudan, Mozambique, Central African Republic, Niger, Chad, Democratic Republic of the Congo, Sudan and Nigeria.
- 3 countries – Ghana, Kenya and Malawi – introduced the first malaria vaccine, RTS,S, in 2019 during the Malaria Vaccine Implementation Programme (MVIP), co-funded by Gavi, the Global Fund to Fight AIDS, Tuberculosis and Malaria and UNITAID. Since 2024, these countries have been funded under Gavi’s Malaria Vaccine Programme, aligning them with the other countries that have introduced malaria vaccines.
Malaria vaccine safety and effectiveness: Both the RTS,S/AS01 and R21/Matrix-M vaccines are prequalified and recommended by WHO to prevent malaria in children and are safe and effective.
- In phase 3 clinical trials, both vaccines reduced malaria cases by more than half during the first year after vaccination, a period when children are at high risk of illness and death. A fourth dose given in the second year of life prolonged protection.
- Both vaccines reduce malaria cases by 75% when given seasonally in areas of highly seasonal transmission.
- The vaccines target P. falciparum, the deadliest malaria parasite globally and the most prevalent in Africa. More about the vaccine here: https://www.who.int/news-room/questions-and-answers/item/q-a-on-rts-s-malaria-vaccinee
Useful information and resources:
- The total burden of malaria in the 20 countries in Africa that are currently vaccinating children accounts for over 70% of the global malaria burden according to the 2024 World Malaria Report.
- Everything you need to know about the malaria vaccine
- Gavi news releases on malaria
- Stories from communities on malaria
- Immunisation and climate adaptation
Read on VaccinesWork
How to apply for vaccine support
Latest articles about malaria
View moreMalaria researchers are getting closer to outsmarting the world’s deadliest parasite
After decades of stalled progress, new vaccines, treatments and genetic tools are helping scientists protect children and save lives worldwide.
Crossing rivers to fight malaria
Grassroots turning the tide against vaccine hesitancy in rural Lilongwe.
Kenya’s Wasini Island may look lost in time, but it’s keeping up on immunisation
Electricity may still be a relatively novel luxury for islanders, but health workers are making sure vaccination is routine and reliable.